Synthesis process of D-sulbenicillin sodium
A technology of sulbenicillin sodium and synthesis process, which is applied in the direction of organic chemistry, etc., can solve the problems of unstable reaction of intermediates, low product yield, long reaction time, etc., to achieve increased product yield, quicker reaction time, and improved yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
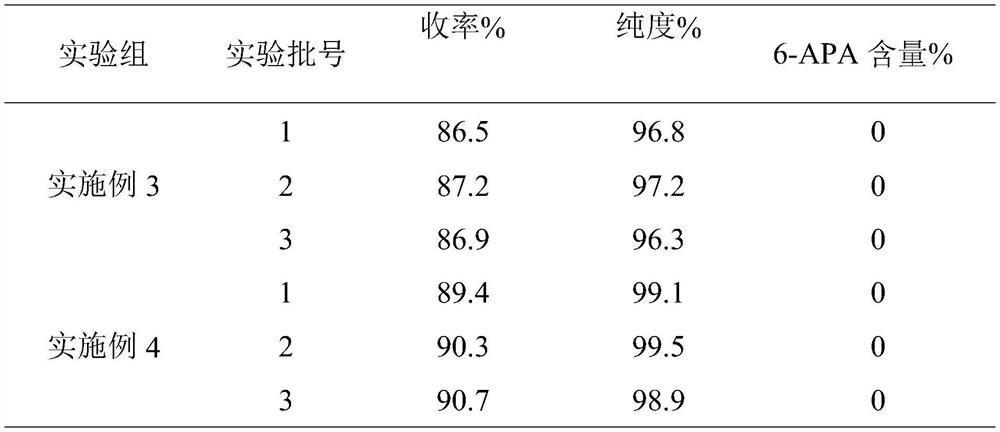
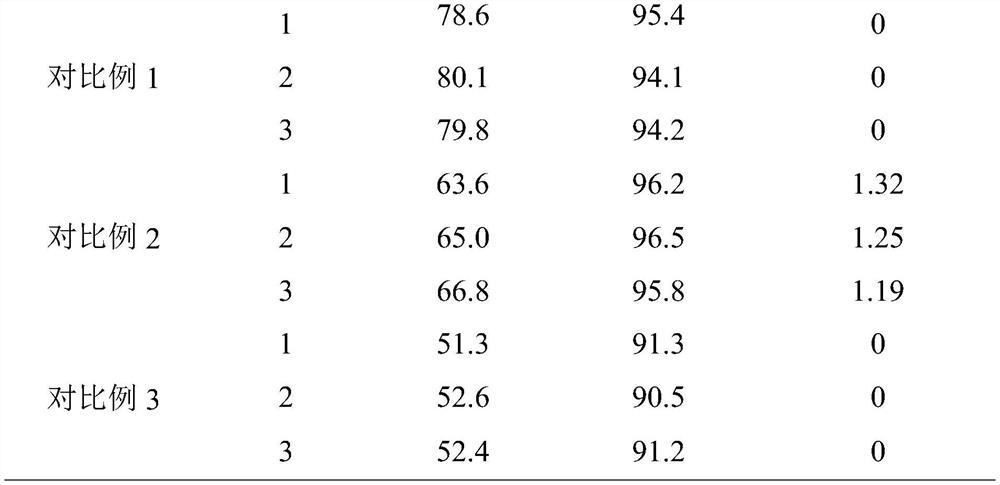
Embodiment 1
[0027] A kind of synthetic technique of D-sulfabenicillin sodium, comprises the following steps:
[0028] S1. Water, ethanol and 2-methyltetrahydrofuran were weighed and mixed according to a volume ratio of 4:4:1 to obtain a mixed solvent;
[0029] S2. Add 6-APA into the mixed solvent and mix, keep the constant temperature at 15°C, add NaOH solution dropwise to adjust the pH to 5.6, then raise the temperature to 23°C and perform ultrasonic dispersion treatment with a power of 150W for 8min, and keep the temperature at 23°C, and the pH is Under the condition of 5.6, the ethyl acetate solution of D-sulfophenylacetyl chloride is added dropwise, wherein, the mass concentration of D-sulfophenylacetyl chloride is 5%, and the mass ratio of 6-APA and D-sulfophenylacetyl chloride is 1: 1., the rate of addition is 1 / 45 of the total amount of the ethyl acetate solution of D-sulfophenylacetyl chloride added dropwise per minute to obtain the mixed initial liquid;
[0030] S3. Put the mixe...
Embodiment 2
[0035] A kind of synthetic technique of D-sulfabenicillin sodium, comprises the following steps:
[0036] S1. Water, ethanol and 2-methyltetrahydrofuran were weighed and mixed in a volume ratio of 4:8:2.5 to obtain a mixed solvent;
[0037] S2. Add 6-APA into the mixed solvent and mix, keep the constant temperature at 20°C, add dropwise NaOH solution to adjust the pH to 5.8, then raise the temperature to 25°C and perform ultrasonic dispersion treatment with a power of 200W for 10min, and keep the temperature at 25°C, and the pH is Under the condition of 5.8, the ethyl acetate solution of D-sulfophenylacetyl chloride is added dropwise, wherein, the mass concentration of D-sulfophenylacetyl chloride is 15%, and the mass ratio of 6-APA and D-sulfophenylacetyl chloride is 1: 1.3, the rate of addition is 1 / 50 of the total amount of the ethyl acetate solution of D-sulfophenylacetyl chloride added dropwise per minute to obtain the mixed initial solution;
[0038] S3. Put the mixed i...
Embodiment 3
[0042] A kind of synthetic technique of D-sulfabenicillin sodium, comprises the following steps:
[0043] S1. Water, ethanol and 2-methyltetrahydrofuran were weighed and mixed according to a volume ratio of 4:6:2.0 to obtain a mixed solvent;
[0044] S2. Add 6-APA to the mixed solvent and mix, keep the constant temperature at 18°C, add NaOH solution dropwise to adjust the pH to 5.7, then raise the temperature to 24°C and perform ultrasonic dispersion treatment with a power of 180W for 9 minutes, and keep the temperature at 24°C, and the pH is Under the condition of 5.7, the ethyl acetate solution of D-sulfophenylacetyl chloride is added dropwise, wherein, the mass concentration of D-sulfophenylacetyl chloride is 10%, and the mass ratio of 6-APA and D-sulfophenylacetyl chloride is 1: 1.15, the rate of addition is 1 / 50 of the total amount of the ethyl acetate solution of D-sulfophenylacetyl chloride added dropwise per minute, to obtain the mixed initial solution;
[0045] S3. Put...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com