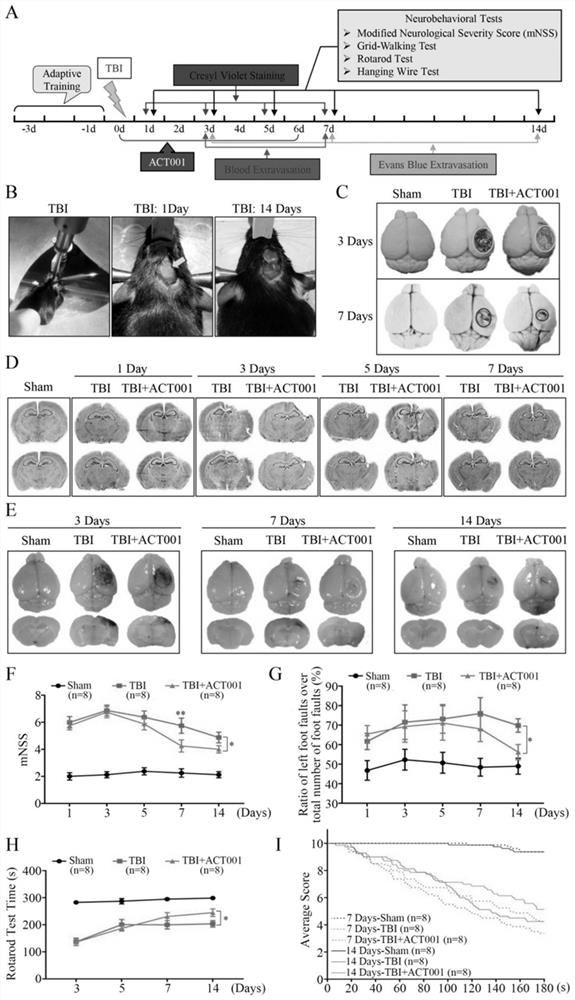
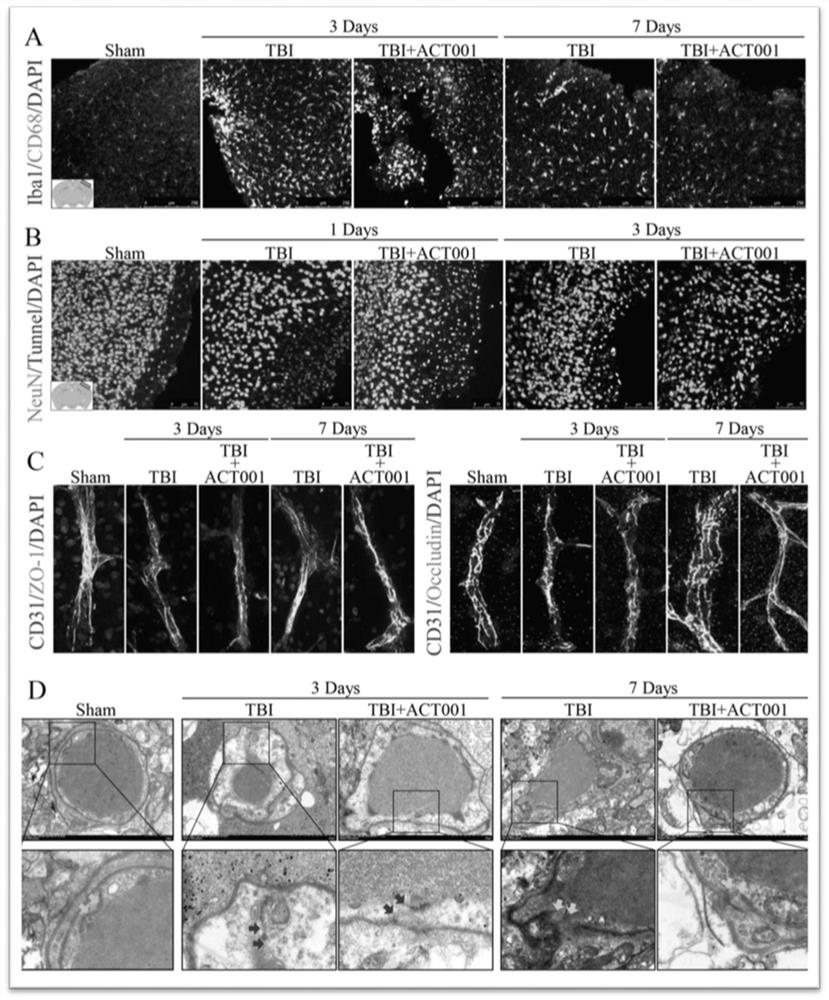
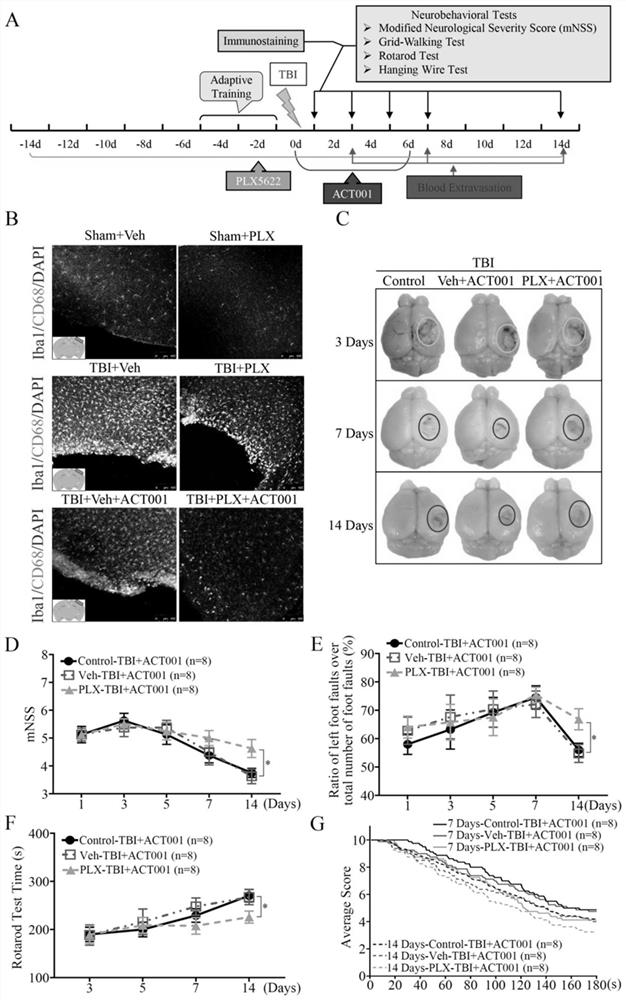
Application of sphaelactone and derivatives thereof in treatment of traumatic craniocerebral injury
A craniocerebral injury and derivative technology, applied in the field of medicine, can solve problems such as restraint
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation of embodiment 1 Milicolactone (MCL)
[0061] Take Taiwan Michelle as raw material, crush it into 20-40 meshes, put it into the extraction kettle, use anhydrous methanol as entrainer, and use supercritical CO 2 Extraction, collect the extract, recover methanol to obtain the extract, reflux with petroleum ether to remove fat-soluble impurities, then heat-dissolve the degreased matter with low-carbon alcohol, add activated carbon to reflux for decolorization, and recover the solvent to an appropriate volume from the decolorization solution. Purify with an ODS RP-C18 reverse-phase column, elute with a mixed solution of methanol and water, concentrate the corresponding components, and recrystallize from petroleum ether-acetone (2:1, V / V) to obtain the MCL product. The supercritical CO 2 The extraction conditions are that the extraction temperature is 45-60°C, the extraction pressure is 23-34MPa, the temperature of the separation tank I is 40-50°C, and the pr...
Embodiment 2
[0062] The preparation of embodiment 2 migna lactone derivatives (ACT001)
[0063] The preparation method of mimilactone derivative (ACT001): the Me 2 NH·HCl (1.5g, 18mmol), K 2 CO 3 (5.0g, 36mmol) and CH 2 Cl 2 (100 ml) were mixed at room temperature and a homogeneous solution was formed. Then MCL (300mg, 1.2mmol) was mixed with the homogeneous solution, and stirred at room temperature for 3h. The above reaction solution was used CH 2 Cl 2 After concentrating and resuspending, use deionized water chromatography to separate, and the obtained separation liquid is separated by Na 2 SO 4 Drain. The filtered material was washed with CH 2 Cl 2 (5ml) was dissolved and treated with fumarate (0.1N) to adjust the pH of the solution to 4. Finally use CH 2 Cl 2 (10ml) to extract the aqueous phase and freeze-dry to obtain compound ACT001. The drug wwACT001 in this experiment was dissolved in deionized water to form a 400mg / ml stock solution, which was then stored in a -80°C...
Embodiment 3
[0064] Example 3 Establishment of Mouse Controlled Cortical Impact (CCI, Controlled Cortical Impact) Model
[0065] Male C57BL / 6J mice (provided by Shanghai Slack Experimental Animal Co., Ltd., 8-10 weeks old, weighing 20-25 g). 0.6% pentobarbital solution, 80mg / kg, intraperitoneally inject anesthetized mouse; Mouse is fixed in brain stereotaxic apparatus (Stoeling Inc.USA); Make a midline incision, separate the scalp, and peel off the periosteum; make a skull burr hole 1.5-2 mm away from the midline between the right coronal suture and herringbone suture, and make a bone window with a diameter of about 4 mm to expose the complete dura mater; apply PinPoint TM PCI3000 fine craniocerebral impactor (Hatteras Instruments Inc. USA) set the impact parameters, and used the impact head with a diameter of 3mm to accurately impact the mouse cerebral cortex. Among them, the strike depth in the experiment is 1.5mm; the strike time is 100ms; the strike speed is 1.5m / s. Bleeding was stop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com