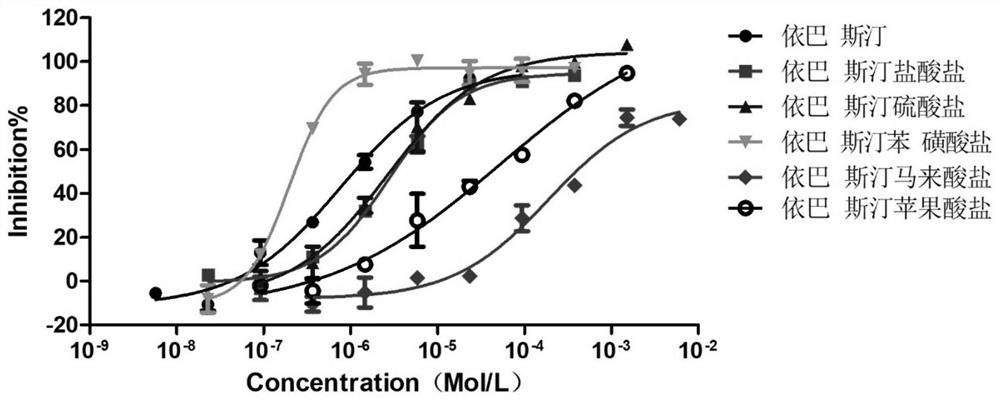
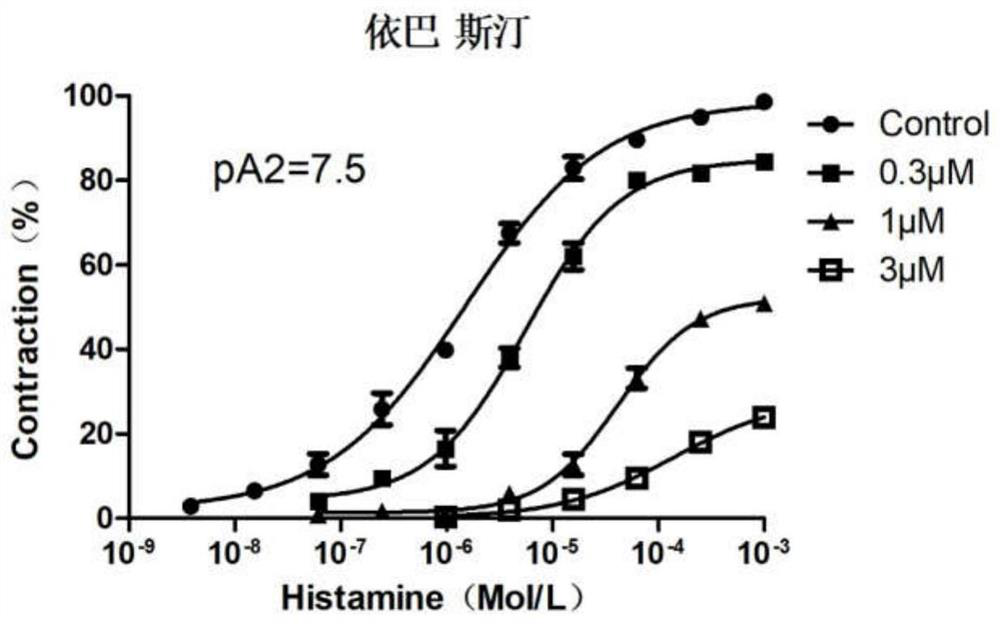
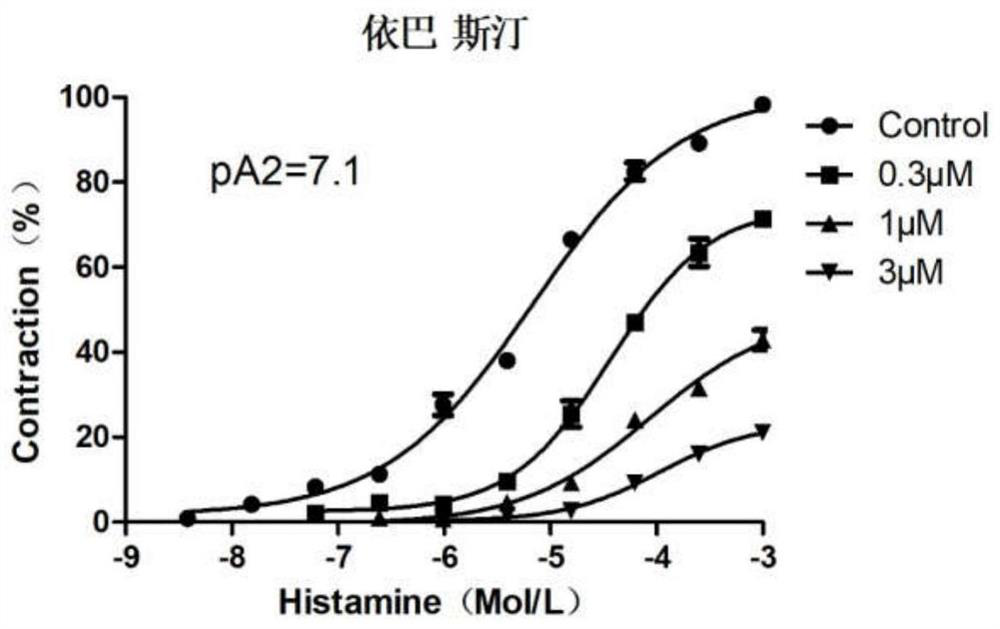
Ebastine salt as well as preparation method and application thereof
A technology of ebastine and hydrochloric acid, applied in the field of pharmacy, can solve the problems of limiting the therapeutic effect of ebastine, poor tablet dissolution, low bioavailability and the like, and achieves simple and convenient post-processing and mild reaction conditions. , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Debas Tatin hydrochloride preparation:
[0035]
[0036] (1) Place the twistin (10.0 g, 21.3 mmol, 1.0 Equiv.) In a 100 ml round bottom flask, ethanol (15 ml). The hydrogen chlorinated hydrogen chlorinated solution (20% in etOh, 5.8 g, 32.0 mm, 1.5 equiv.) Was dripped into the twistin solution at room temperature at 25 ° C, and the solution gradually became clarified. After completion of the boss, stir it for 10 min, evaporate the solvent under reduced pressure, and gelatin hydrochloric acid was crude according to Bastine;
[0037] (2) The hydrochloric acid obtained in step (1) is added to hexane (15 ml), heating and reflow, gradually incorporating acetate (55 mL), continued to reflow for 1 h, then naturally cooled to room temperature and stir over night Filtration, filter cake was washed with EtOAc (EtOAc) The purity is 97.32%;
[0038] The identification data of the bisenine hydrochloride has been subjected to as follows:
[0039] 1 H NMR (500MHz, DMSO-D 6 : δ 10.82 (1H...
Embodiment 2
[0043] Debarst sulfate preparation:
[0044]
[0045](1) The ebastine (10.0g, 21.3mmol, 1.0equiv.) Was placed in a 100mL round bottomed flask was added acetone (15 mL), stirred at room temperature 25 ℃ Sulfuric acid (98%, 2.3g, 22.4mmol, 1.05 equiv.) was dissolved in acetone (5mL) was added dropwise, and the solution was heat gradually became clear. After the addition is complete stirring was continued for 10min, the solvent was evaporated under reduced pressure to give a crude gum sulfate ebastine;
[0046] (2) Sulfuric acid (1) obtained in hexane was added (15mL) to step ebastine crude product, heated to reflux, was added dropwise gradually ethyl acetate (20 mL), heating was continued at reflux IH, cooled to room temperature followed by stirring, the filter cake with hexane / ethyl acetate: washing under (1 1,5mL × 3) 60 ℃ dried 3h, to give 9.60 g of white solid sulfate ebastine, yield 79.6%, purity according to HPLC test results show to 97.67%;
[0047] Identification data ob...
Embodiment 3
[0052] Benzenesulfonate prepared by Bastin
[0053]
[0054] (1) The ebastine (10.0g, 21.3mmol, 1.0equiv.) Was placed in a 100mL round bottom flask was added ethanol (20 mL), a clear solution was heated to 50 deg.] C, stirring acid (3.5g, 22.4mmol, 1.05equiv.) was dissolved in ethanol (10 mL) was added dropwise. After the addition is complete stirring was continued for 10min, the solvent was evaporated under reduced pressure to give the crude acid ebastine;
[0055] (2) to step (1) obtained in the crude acid ebastine added acetone (15 mL), dissolved was heated to 80 deg.] C, cooled to 0 deg.] C with stirring, a white solid was precipitated, stirring was continued for 1h. Filtered, washed, the filter cake with acetone (5mL × 3) 60 ℃ dried 3h, ebastine acid as a white solid 11.62 g, yield 87.4%, according to HPLC results showed a purity of 98.89%;
[0056] Identification data obtained by ebastine benzenesulfonate follows:
[0057] 1 H NMR (500MHz, DMSO-d 6 ): Δ9.03 (1H, s), 7.91-7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com