Synthesis method of cefcapene acid
A technology of cefcapine acid and a synthesis method, which is applied in the field of synthesis of cefcapine acid, can solve the problems of little research on cefcapine acid, many impurities and low purity of cefcapine acid, and achieves a simple, efficient, and deoxidizing method. The effect of high removal efficiency and low environmental protection pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a kind of synthetic method of cefcapene acid, comprises the following steps:
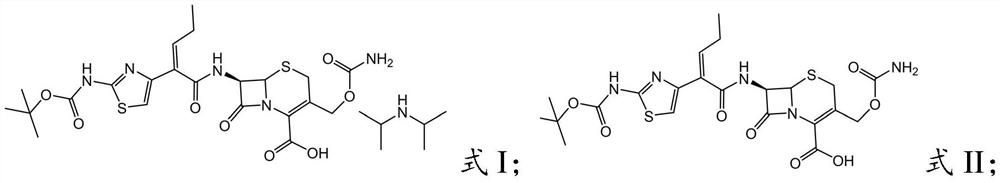
[0030] (1) BCN, organic solvent and inorganic acid are mixed to carry out acidification reaction, and gained acidification feed liquid is left standstill layered, obtains the organic phase that contains desalination BCN; Described BCN is the precursor acid of cefcapene pivoxil, and structural formula is as formula I Shown; The structural formula of described desalted BCN is shown in formula II:
[0031]
[0032] (2) The organic phase containing desalted BCN is mixed with an acid to perform a tert-butoxycarbonyl removal reaction, and the resulting product is subjected to solid-liquid separation to obtain a crude product of cefcapene acid;
[0033] (3) dispersing the crude product of cefcapene acid in water, adjusting the pH value of the obtained dispersion to 8.0-9.0 with alkali, and then performing solid-liquid separation to obtain a filtrate;
[0034] (4) adjusting...
Embodiment 1
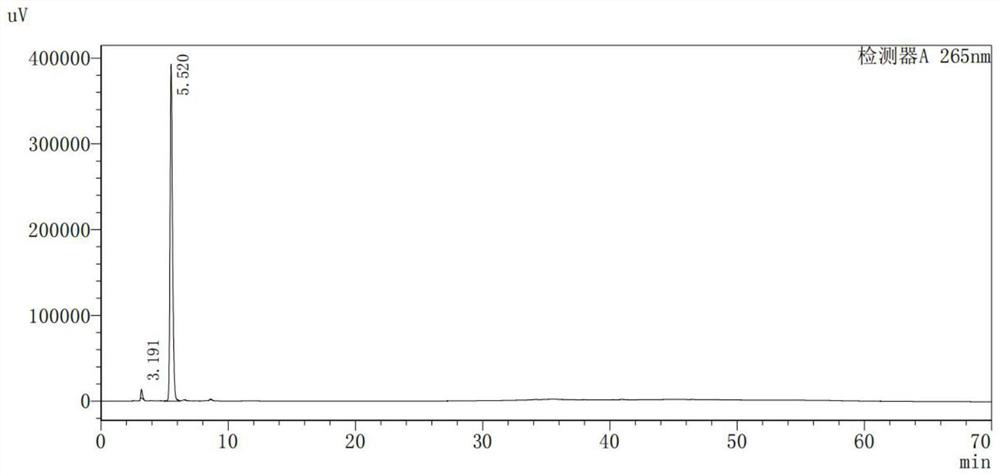
[0047] At 25°C, add 10 g of crude BCN and 100 mL of dichloromethane to the flask. After stirring evenly, add dilute hydrochloric acid dropwise until the pH value is 1.5. Stir until the system dissolves. Add 20 mL of concentrated hydrochloric acid dropwise, crystals are obviously precipitated, and react for 60 minutes; filter and wash the filter cake with 20 mL of pure water; at 25°C, add the filter cake and 100 mL of pure water to the flask, stir well, then add 7% carbonic acid dropwise Sodium hydrogen solution, adjust the pH value to 8, stir for 30 minutes, filter, and collect the filtrate; add 3.6% hydrochloric acid dropwise to the filtrate, adjust the pH value to 2, precipitate crystals, cool down to 5°C, grow crystals for 60 minutes, filter, and drain , transferred to an oven at 30° C. and dried under reduced pressure for 3 hours; 6.7 g of dry product was obtained, and the product purity was 98.4%. The HPLC detection data of product are shown in Table 1, and the HPLC chrom...
Embodiment 2
[0051] At 25°C, add 10 g of crude BCN and 100 mL of ethyl acetate to the flask, stir evenly, then add dilute hydrochloric acid dropwise until the pH value is 2.0, stir until the system dissolves, let stand to separate layers, and then pour into the ethyl acetate layer Add 20 mL of concentrated hydrochloric acid dropwise, crystals are obviously precipitated, and react for 60 minutes; filter and wash the filter cake with 20 mL of pure water; at 25°C, add the filter cake and 100 mL of pure water to the flask, stir well, then add 7% carbonic acid dropwise Sodium hydrogen solution, adjust the pH to 9, stir for 30 minutes, filter, and collect the filtrate; add 3.6% hydrochloric acid dropwise to the filtrate, adjust the pH to 4, precipitate crystals, cool down to 5°C, grow crystals for 60 minutes, filter, and drain , transferred to an oven for drying under reduced pressure at 30° C. for 5 h; 5.5 g of dry product was obtained, and the product purity was 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com