Amino-containing polyarylether polymer as well as preparation method and application thereof
An amino polyarylene ether and polymer technology, applied in the field of amino-containing polyarylene ether polymers and their preparation, can solve the problems of membrane pollution, high cost, incompetence, etc., and achieve broad application prospects, good solvent resistance, good interaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The present invention also provides a method for preparing the amino group-containing polyarylether polymer described in the above technical solution, comprising the following steps:
[0061] a) under the action of a base catalyst, carry out nucleophilic polycondensation of amino-containing diphenol monomers and aromatic dihalogen monomers of the structure shown in formula (8) in a polar aprotic solvent to obtain amino-containing polyarylethers thing;
[0062]
[0063] In the present invention, the aromatic dihalogen monomer preferably has the structures shown in formulas (9) to (11):
[0064]
[0065] Wherein, X represents halogen, preferably F and / or Cl. In the present invention, there is no special limitation on the source of the aromatic dihalogen monomer, and commercially available products well known to those skilled in the art can be used.
[0066] In the present invention, the preparation method of the amino-containing diphenol monomer of the structure s...
Embodiment 1
[0108] (1) Preparation of amino-containing diphenol monomers:
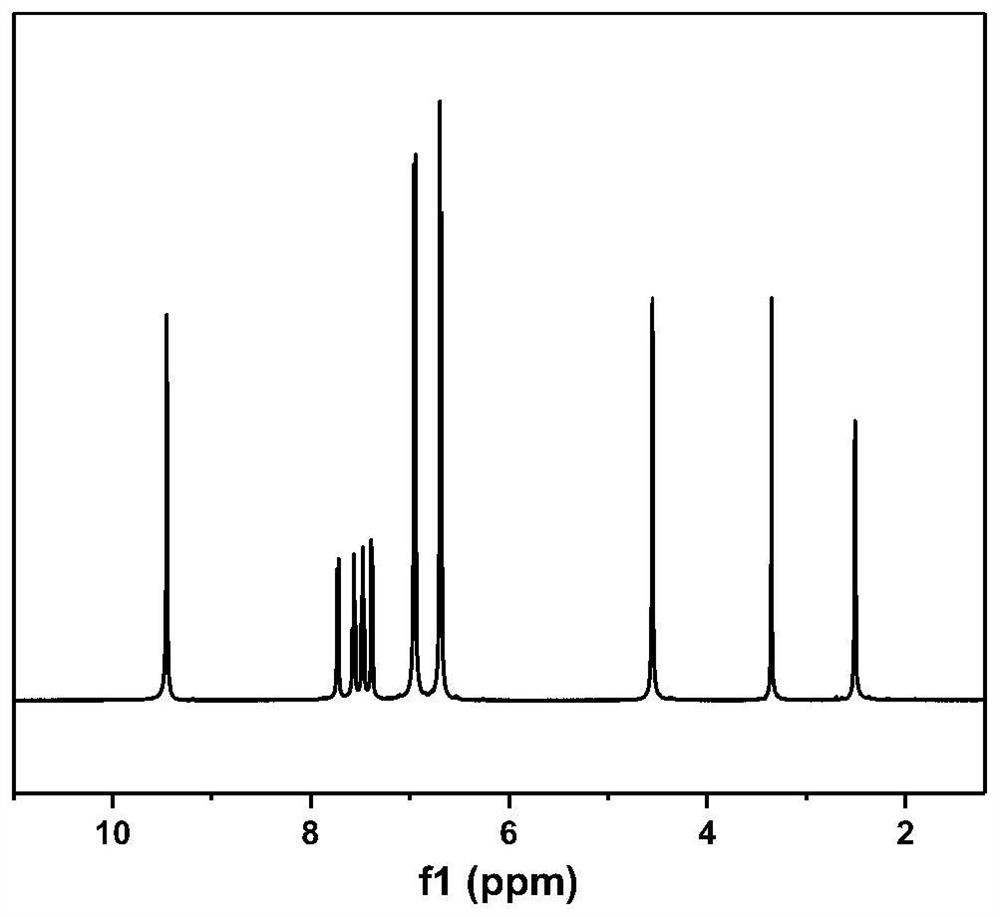
[0109] Add 31.83g of phenolphthalein and 62.5g of 40wt% hydrazine hydrate aqueous solution into a single-necked bottle, and react at 60°C for 12 hours under magnetic stirring, the system turns from purple to white, and finally becomes a white turbid liquid; a white solid is obtained by filtration, and washed with a large amount of deionized water , recrystallized with methanol after drying to obtain the amino group-containing diphenol monomer with the structure shown in formula (8). Its H NMR spectrum is as figure 1 shown. The reaction formula is as follows:
[0110]
[0111] (2) Preparation of amino-containing polyaryl ether ketone:
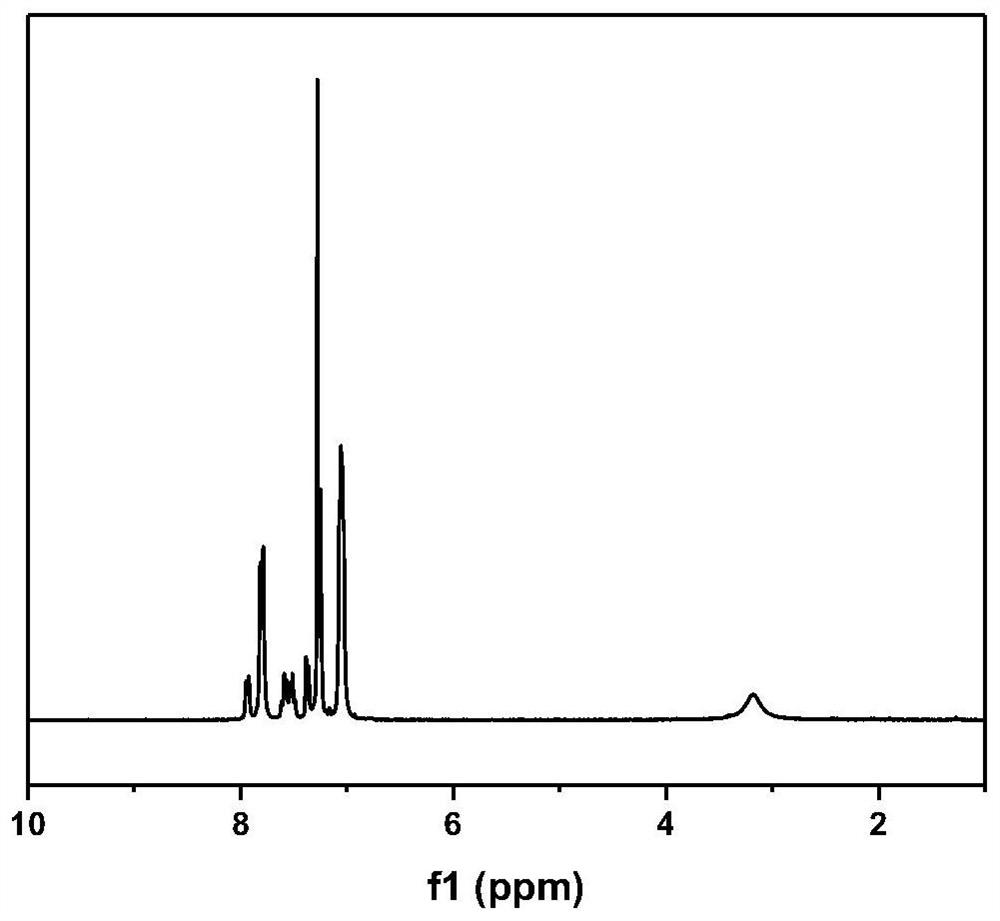
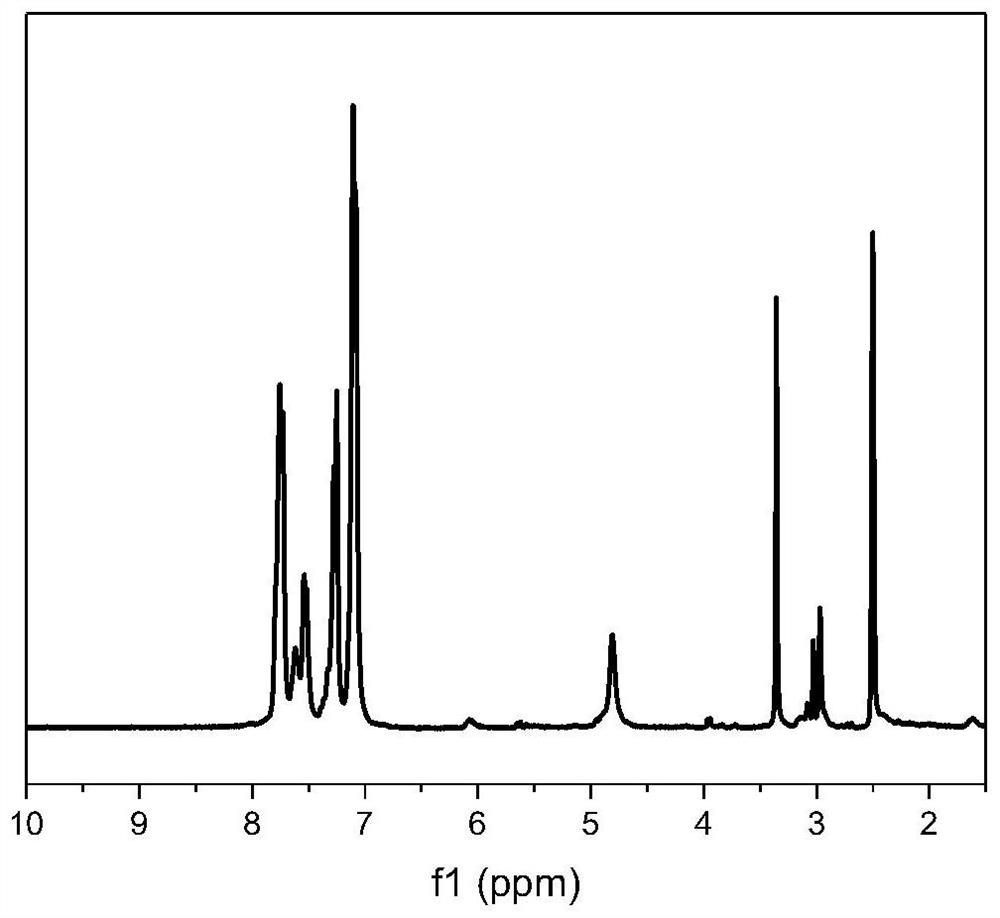
[0112] 16.62g (0.05mol) of the amino-containing diphenol monomer prepared according to the above step (1), 10.91g (0.05mol) of 4,4'-difluorobenzophenone, 14.16g (0.1025mol) of anhydrous carbonic acid Potassium, 40ml of toluene and 100ml of dimethyl sulfoxide were added to a t...
Embodiment 2
[0114] (1) Preparation of amino-containing diphenol monomers:
[0115] See Example 1.
[0116] (2) Preparation of amino-containing polyaryl ether ketone:
[0117] 16.62g (0.05mol) of the amino-containing diphenol monomer prepared according to the above step (1), 10.91g (0.05mol) of 4,4'-difluorobenzophenone, 8.98g (0.065mol) of anhydrous carbonic acid Potassium, 50ml of N-methylpyrrolidone was added to a three-neck flask containing mechanical stirring, nitrogen inlet, water separator and condenser tube, and heated to 200°C for 4 hours under nitrogen atmosphere to obtain a viscous polymer solution. Add 200ml of N- After dilution with methylpyrrolidone, the product is poured into deionized water, and the solid is washed and dried to obtain amino-containing polyaryletherketone. Its structure is shown in formula (6), wherein, n=100.
[0118] Embodiment 1 step (2) and embodiment 2 step (2) reaction formula are as follows:
[0119]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com