A kind of detection method of related substance in rifampicin
A detection method and a technology for related substances, which are applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that there are no relevant literature reports on the detection methods of N-methylpiperazine and 1-amino-4-methylpiperazine, etc. Achieve the effect suitable for practical application and promotion, good linear relationship and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
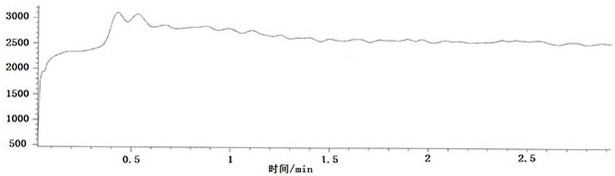
[0045] The embodiment of the present application provides a method for detecting a related substance in rifampicin, and the method for detecting comprises the following steps:
[0046] Step 1. Prepare blank solution, test solution, reference solution and 100% test solution fortification:
[0047] The blank solution is acetonitrile solvent;
[0048] Preparation of the test solution: take 50mg of rifampicin sample, accurately weigh it, place it in a 5mL volumetric flask, add acetonitrile to dissolve and dilute to the mark, shake well, and use it as the test solution;
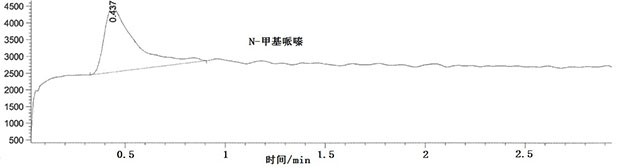
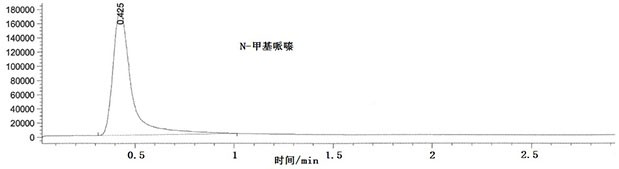
[0049] Preparation of reference substance stock solution: respectively take 62 mg of N-methylpiperazine reference substance and 1-amino-4-methylpiperazine reference substance, accurately weigh them, put them in a 50mL volumetric flask, add acetonitrile to dissolve and dilute to the mark , shake well; then transfer 1.0mL of the above solution to a 50mL volumetric flask, add acetonitrile to dilute to the mark, shak...
Embodiment 2
[0060] Example 2 Repeatability
[0061] The repeatability of N-methylpiperazine: take the same batch of samples, prepare 6 parts of 100% test sample spiked solutions in parallel, and use liquid chromatography-mass spectrometry to detect. The specific conditions of liquid chromatography and mass spectrometry are as described in Example 1, and the results are shown in Table 1.
[0062] Wherein, the preparation method of the above-mentioned 100% test substance spiked solution is: take about 50 mg of rifampicin sample, accurately weigh it, place it in a 5mL volumetric flask, accurately add 2.0mL reference substance stock solution, and then add acetonitrile to make the volume up to the mark , Shake well, as 100% test sample spiked solution.
[0063] The repeatability of 1-amino-4-methylpiperazine: take the same batch of samples, prepare 6 parts of 100% spiked solution of the test product in parallel, and use liquid chromatography-mass spectrometry for detection. The specific chara...
Embodiment 3
[0068] Example 3 intermediate precision
[0069] Intermediate precision solution: take the same batch of samples, prepare 6 parts of 100% standard spiked solution in parallel, and use different instruments in the same laboratory at different times for liquid chromatography-mass spectrometry detection. The conditions are as described in Example 1, and the results are shown in Table 2.
[0070] The preparation method of above-mentioned 100% need testing product standard addition solution is: get about 40mg of rifampicin sample, weigh accurately, place 5mL volumetric flask, accurately add 2.0mL reference substance stock solution, then add acetonitrile to be settled to scale, shake Uniformly, as 100% test sample spiked solution.
[0071] Table 2 Intermediate precision results
[0072]
[0073] It can be seen from Table 2 that the RSD of the content of each analyte in the 6 intermediate precision solutions is less than or equal to 10.0%, indicating that the intermediate precis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com