Double-layer artificial periosteum, preparation method and application thereof
An artificial bone, double-layer technology, applied in the fields of medical science, prosthesis, tissue regeneration, etc., can solve the problem that the periosteum cannot effectively inhibit osteosarcoma bone cancer, etc., and achieve the effect of preventing the growth into the bone defect area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
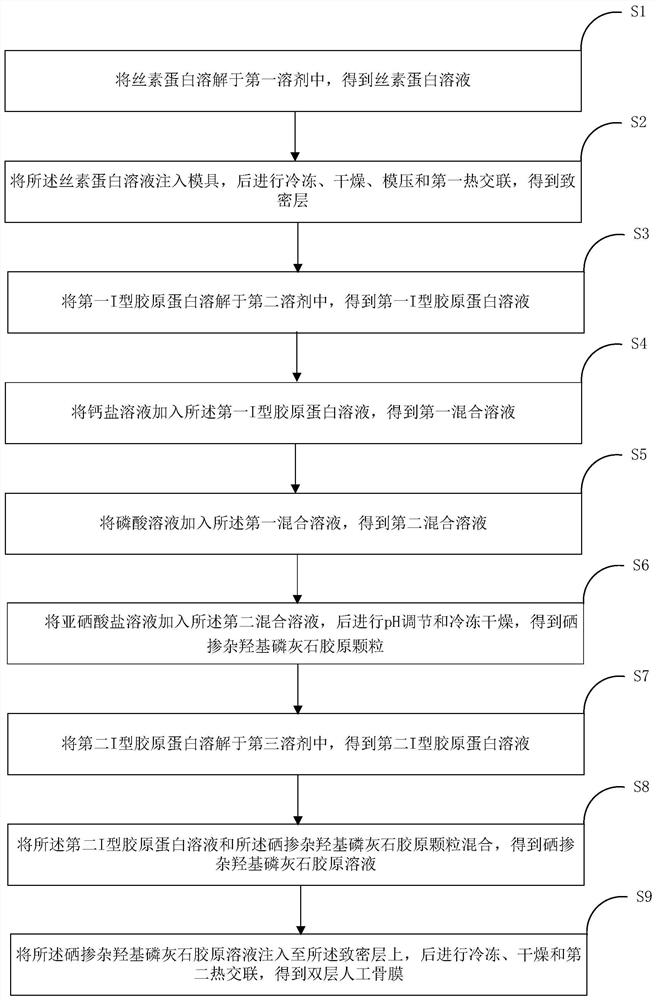
[0048] According to a typical implementation of the present invention, a method for preparing a double-layer artificial periosteum is provided, the method comprising:
[0049] S1, the preparation of dense layer, specifically include:
[0050] S1-1. Dissolving silk fibroin in lithium bromide solution or calcium chloride ternary system (calcium chloride / water / ethanol molar ratio 1:8:2) solution to obtain silk fibroin with a mass fraction of 5% to 20% protein solution.
[0051] The silk fibroin is selected from the natural high-molecular fiber protein extracted from silkworm cocoons or silk, and the mass fraction of silk fibroin in the silk fibroin solution is 5% to 20%. The reason: too high mass fraction of silk fibroin is not conducive to Degradation is slow during dissolution and use, and the low mass fraction affects the mechanical properties and physical barrier function of the membrane;
[0052] S1-2, taking the silk fibroin solution obtained in step S1-1 and injecting it...
Embodiment 1
[0094] A preparation method of double-layer artificial periosteum, the method comprising:
[0095] S1, the preparation of dense layer, specifically include:
[0096] S1-1. Dissolving silk fibroin in lithium bromide solution or calcium chloride ternary system (calcium chloride / water / ethanol molar ratio 1:8:2) solution to obtain a silk fibroin solution with a mass fraction of 5%.
[0097] S1-2. Take the silk fibroin solution obtained in step S1-1 and inject it into the mold, pre-freeze at -60°C for 12 hours, then dry the frozen body at a temperature of 10°C and a pressure of 10Pa for 48 hours, and then mold it under a pressure of 20MPa 60s to obtain dense layer film;
[0098] Thermally cross-linking the dense layer film at 110° C. for 24 hours in a vacuum oven to obtain a dense layer;
[0099] S2, preparation of selenium-doped hydroxyapatite collagen solution, specifically including:
[0100] S2-1. Dissolving type I collagen in an acetic acid solution with a mass fraction of ...
Embodiment 2
[0111] A preparation method of double-layer artificial periosteum, the method comprising:
[0112] S1, the preparation of dense layer, specifically include:
[0113] S1-1. Dissolving silk fibroin in lithium bromide solution or calcium chloride ternary system (calcium chloride / water / ethanol molar ratio 1:8:2) solution to obtain a silk fibroin solution with a mass fraction of 5%.
[0114] S1-2. Take the silk fibroin solution obtained in step S1-1 and inject it into the mold, pre-freeze at -60°C for 12 hours, then dry the frozen body at a temperature of 10°C and a pressure of 10Pa for 48 hours, and then mold it under a pressure of 20MPa 30s to obtain dense layer film;
[0115] Thermally cross-linking the dense layer film at 110° C. for 24 hours in a vacuum oven to obtain a dense layer;
[0116] S2, preparation of selenium-doped hydroxyapatite collagen solution, specifically including:
[0117] S2-1. Dissolving type I collagen in an acetic acid solution with a mass fraction of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com