Preparation method of graphite carbon nitride axially coordinated iron phthalocyanine composite material
A technology of graphitic carbon nitride and composite materials, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc. , easy to aggregate and other problems, to achieve the effects of controllable and easy-to-control production conditions, improved removal rate and decomposition rate, and strong catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
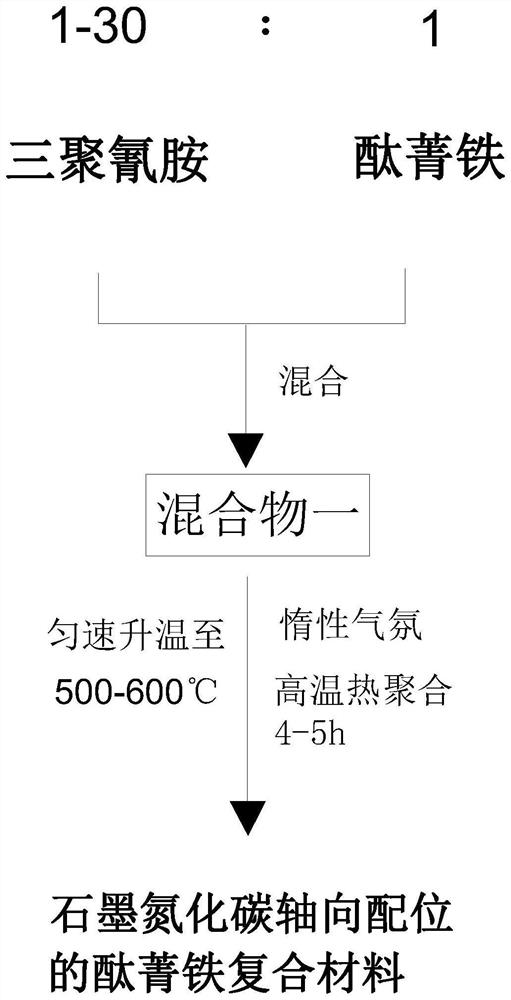
[0037] The present embodiment provides a kind of preparation method of the iron phthalocyanine composite material of graphite carbon nitride axial coordination, such as figure 1 As shown, it includes the following process: prepare iron phthalocyanine and melamine as raw materials according to the mass ratio of 1:(1-30), and mix the two evenly by ball milling; Pyrolysis at high temperature for 4-5 hours; after the pyrolysis is completed, naturally cool to room temperature, and the obtained product is the required graphite carbon nitride axially coordinated iron phthalocyanine composite material.
[0038] Among them, melamine is selected as melamine. The high-temperature pyrolysis process is completed in a tube furnace, and the inert gas atmosphere is nitrogen or helium. The ball milling and mixing process of raw materials is completed in a ball mill, and the ball milling time is not less than 1 hour.
[0039] In order to obtain a purer product, after the calcined product is c...
Embodiment 1
[0042] According to the ratio of parts by mass of 1:1, 5 g of iron phthalocyanine and 5 g of melamine were weighed; the two were fully ball-milled and mixed for 2 hours to obtain a mixed material.
[0043] Put the mixed material obtained in the above step into a tube furnace fed with nitrogen gas, and the tube furnace raises the furnace temperature to 500 °C at a heating rate of 5 °C / min; keeps the pyrolysis reaction for 4 hours, and then gradually lowers the furnace temperature After reaching room temperature, the high-temperature pyrolysis product was taken out, and the product was in the form of black powder.
[0044] Wash and dry the black product in the previous step with methanol and deionized water in sequence to remove soluble impurities therein to obtain a pure graphitic carbon nitride axially coordinated iron phthalocyanine composite material. The product in this example is named FP / CN 1 After detection, the mass fraction of iron element in the material is 7.58%.
Embodiment 2
[0046] According to the ratio of parts by mass of 1:5, 5 g of iron phthalocyanine and 25 g of melamine were weighed; the two were fully ball-milled and mixed for 2 hours to obtain a mixed material.
[0047] Put the mixed material obtained in the above step into a tube furnace fed with nitrogen gas, and the tube furnace raises the furnace temperature to 500 °C at a heating rate of 5 °C / min; keeps the pyrolysis reaction for 4 hours, and then gradually lowers the furnace temperature After reaching room temperature, the high-temperature pyrolysis product was taken out, and the product was in the form of black powder.
[0048] Wash and dry the black product in the previous step with methanol and deionized water in sequence to remove soluble impurities therein to obtain a pure graphitic carbon nitride axially coordinated iron phthalocyanine composite material. The product in this example is named FP / CN 5 After testing, the mass fraction of iron element in the material is 3.49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com