Agomelatine transdermal patch as well as preparation method and application thereof
A transdermal patch and patch technology, applied in the field of medicine, can solve problems such as low bioavailability, and achieve the effects of high bioavailability, good stability and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
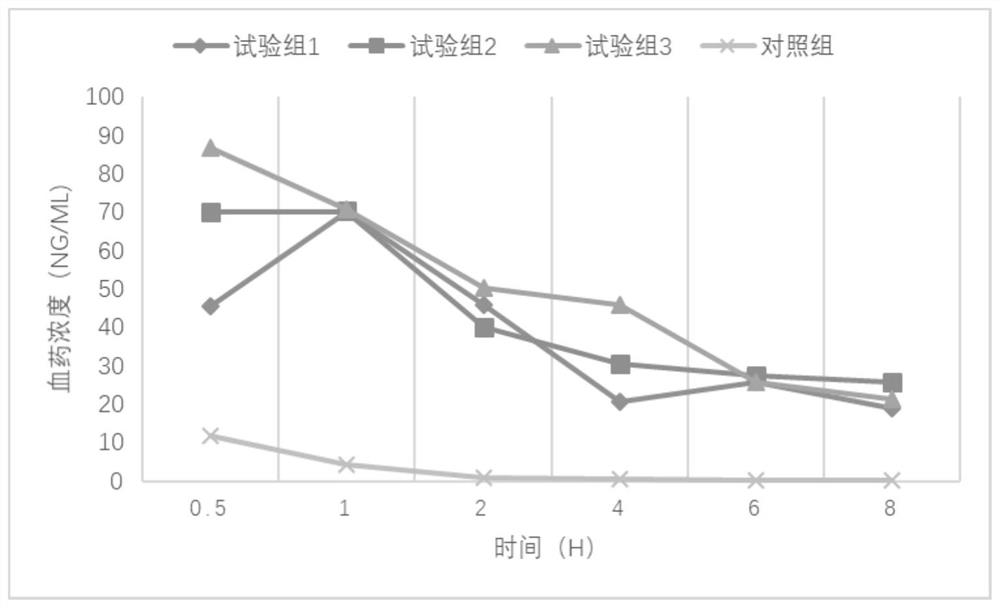
Examples
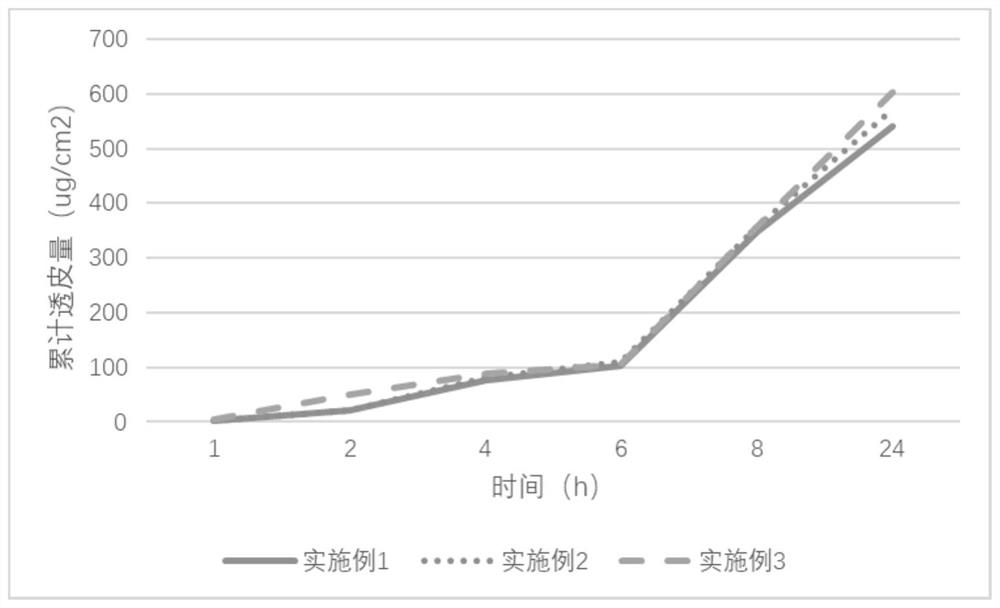
Embodiment 1
[0075] The preparation of embodiment 1 agomelatine transdermal patch
[0076] Composition of agomelatine transdermal patch:
[0077]
[0078] The preparation method of agomelatine transdermal patch comprises the following steps:
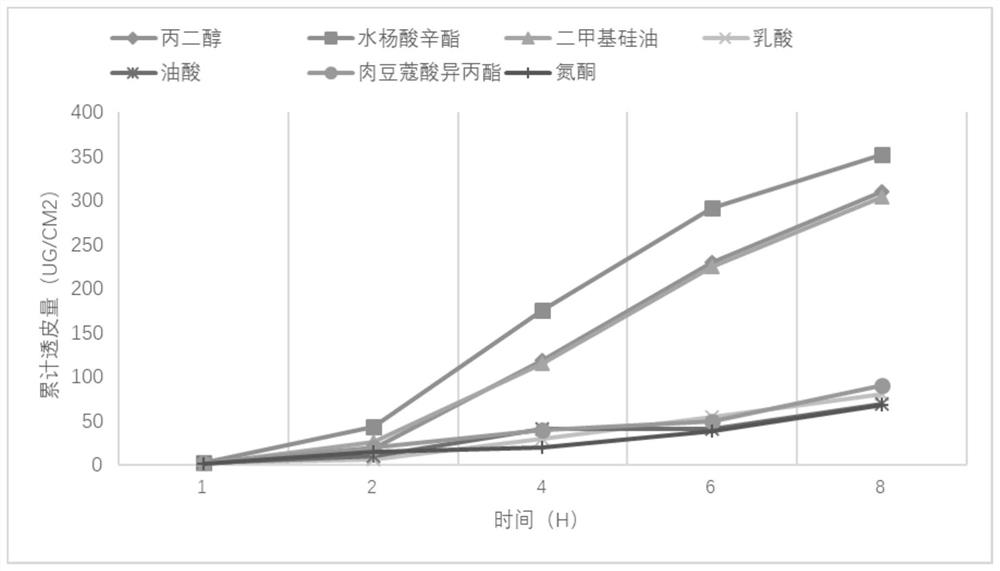
[0079] 1) Weigh the required amount of agomelatine and add it to ethanol, stir at 25°C and 200rpm for 20min, then add octyl salicylate, N-methylpyrrolidone, vitamin E, polyacrylate-13, 25°C , Stirring at 200rpm for 20min to obtain a mixture;
[0080] 2) Spread the mixture on non-woven fabric or tape, cut into 2.2cm 2 (containing 2 mg agomelatine), put it in a drying oven, cure at 60°C for 30 minutes, take it out and apply an anti-adhesive layer, seal it, and get it.
Embodiment 2
[0081] The preparation of embodiment 2 agomelatine transdermal patch
[0082] Composition of agomelatine transdermal patch:
[0083]
[0084] The preparation method of agomelatine transdermal patch comprises the following steps:
[0085] 1) Weigh the required amount of agomelatine and add it to ethanol, stir at 25°C and 200rpm for 20min, then add dimethyl sulfoxide, dimethylacetamide, xanthan gum, BHT in turn, at 25°C and 200rpm Stir for 20min to obtain a mixture;
[0086] 2) Spread the mixture on non-woven fabric or tape, and cut into 2.2cm 2 (containing 4 mg agomelatine), put it in a drying oven, solidify at 80°C for 30 minutes, take it out and apply an anti-adhesive layer, seal it, and get it.
Embodiment 3
[0087] The preparation of embodiment 3 agomelatine transdermal patch
[0088] Composition of agomelatine transdermal patch:
[0089]
[0090] The preparation method of agomelatine transdermal patch comprises the following steps:
[0091] 1) Weigh the required amount of agomelatine and add it to ethyl acetate, stir at 25°C and 200rpm for 20min, then add octyl salicylate, liquid paraffin, simethicone, BHT, polyisobutylene in sequence, at 25°C, Stir at 200rpm for 20min to obtain a mixture;
[0092] 2) Spread the mixture on non-woven fabric or tape, and cut into 2.2cm 2 (containing 4 mg agomelatine), put it in a drying oven, cure at 70°C for 10 minutes, take it out and apply an anti-adhesive layer, seal it, and get it.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com