Slow-release gel drug delivery system for postoperative intracavitary chemotherapy/immune co-treatment as well as preparation method and application of sustained-release gel drug delivery system
A sustained release and drug delivery technology, applied in the field of medicine, can solve the problems of unfavorable clinical transformation application, poor prognosis, and insufficient survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
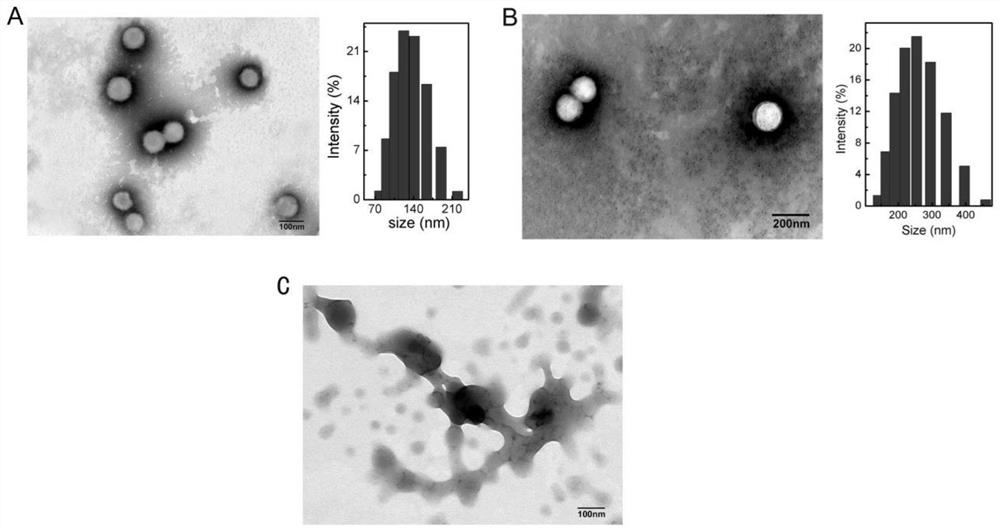
[0066] (1) PSNPs, NPs PTX and PNP PTX Preparation and synthesis
[0067] Weigh 5mg of PTX-SS-C 18 Dissolve in 0.5mL of absolute ethanol to obtain the organic phase. Slowly drop the organic phase into 10mL of pure water (water phase) under constant stirring (700rpm) with a micro syringe at room temperature. The residual organic solvent was removed by rotary evaporation for 5 min, and finally filtered through a 0.45 μm, 0.22 μm microporous membrane to obtain self-assembled PTX prodrug nanoparticles (PSNPs). MeO-PEG 2000 -DSPE and Pep-PEG 2000 -DSPE was dissolved in water respectively, and mixed with the PSNPs prepared by the above method (MeO-PEG 2000 -DSPE or Pep-PEG 2000 -DSPE / PSNPs=20 / 100,w / w), resulting in non-targeting nanoparticles (NP PTX ) and targeted nanoparticles (PNP PTX ).
[0068] Will PSNPs, NP PTX and PNP PTX After diluting to appropriate multiples with deionized water, the particle size was measured with a Malvern laser particle size analyzer Zetasize...
Embodiment 2
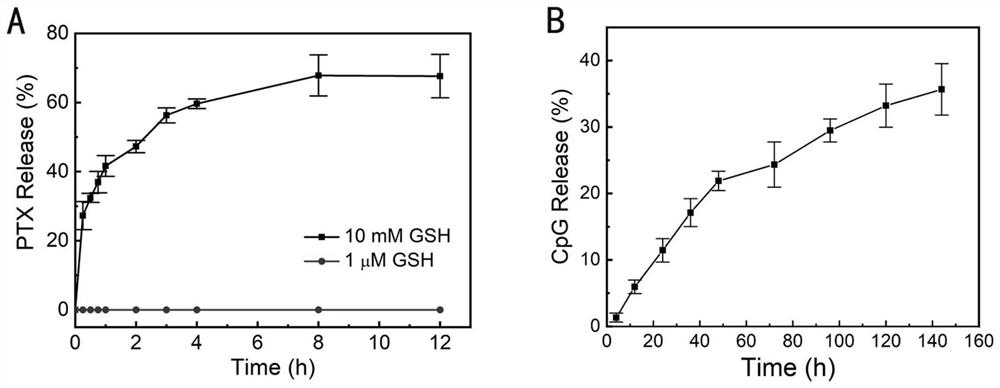
[0080] Example 2PNP PTX and MNP CpG in vitro release of
[0081](1) According to the preparation method of the PTX nanoparticles in the example, the in vitro release behavior of PTX in the nanoparticles was investigated by ultrafiltration centrifugation. Take an appropriate amount of the prepared nanoparticle solution, dilute it to 30mL with release medium, put it in a 50ml EP tube, and the total amount of PTX is 15μg, so as to ensure that the drug release is carried out under the sink condition. The release media were HAc-NaAc buffer (0.04M, pH 5.6) containing 10mM GSH and 0.5% Tween-80 to simulate the tumor microenvironment, and cerebrospinal fluid (CSF, pH 5.6) containing 1 μM GSH and 0.5% Tween-80, respectively. 7.4) to simulate the microenvironment of cerebrospinal fluid. Put the diluted nanoparticles into a 37°C constant temperature shaker (150rpm), take out three groups of samples in parallel at 0.25, 0.5, 0.75, 1, 2, 3, 4, 8, and 12h, and immediately transfer them t...
Embodiment 3
[0084] will PNP PTX and MNP CpG Add to the temperature-sensitive gel solution, the volume ratio of the three is 1:1:8, PNP PTX and MNP CpG The concentration in the temperature-sensitive gel solution is 10%, and the mixture is evenly stirred to obtain the drug-loaded nanogel PNP PTX &MNP CpG @Gel; a temperature sensitive gel solution is prepared by weighing out an amount of PLGA 1750 –PEG 1500 –PLGA 1750 , uniformly dispersed in distilled water, and placed at room temperature for 24 hours to fully swell to obtain PLGA 1750 –PEG 1500 –PLGA 1750 The concentration is 10-30% (w / v) gel aqueous solution, and the concentration is 20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com