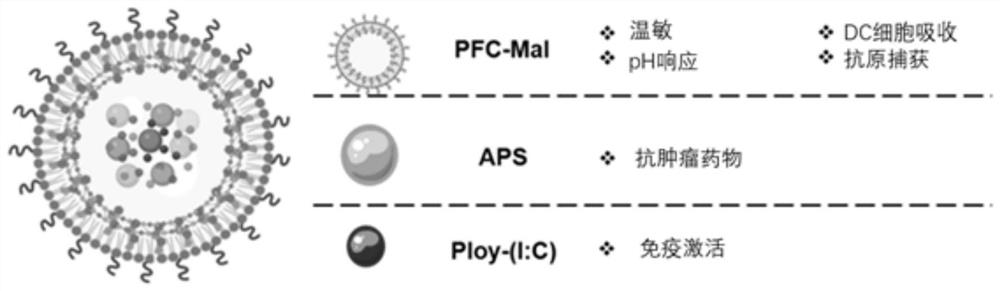
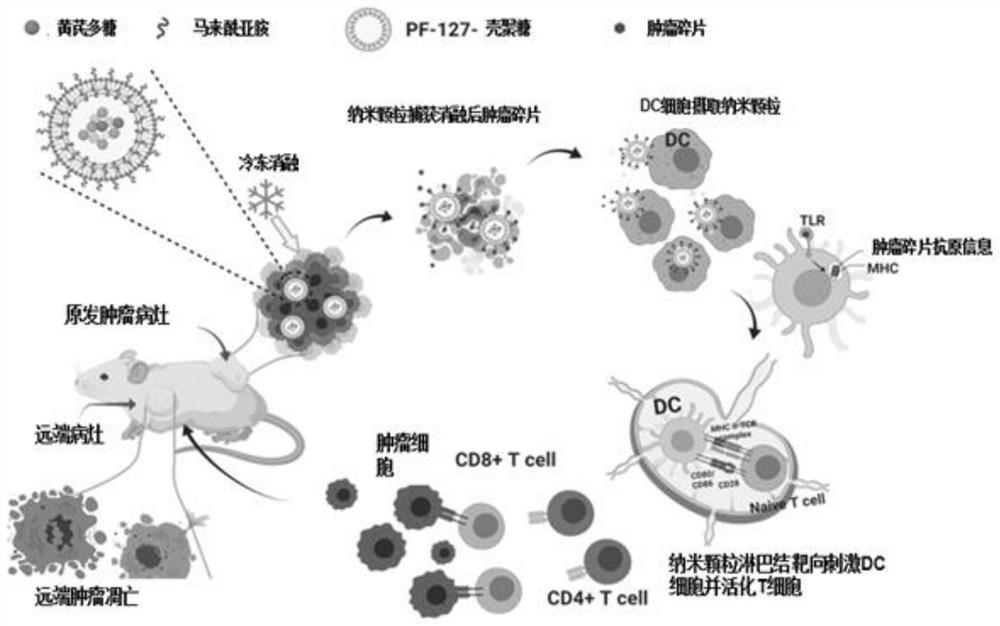
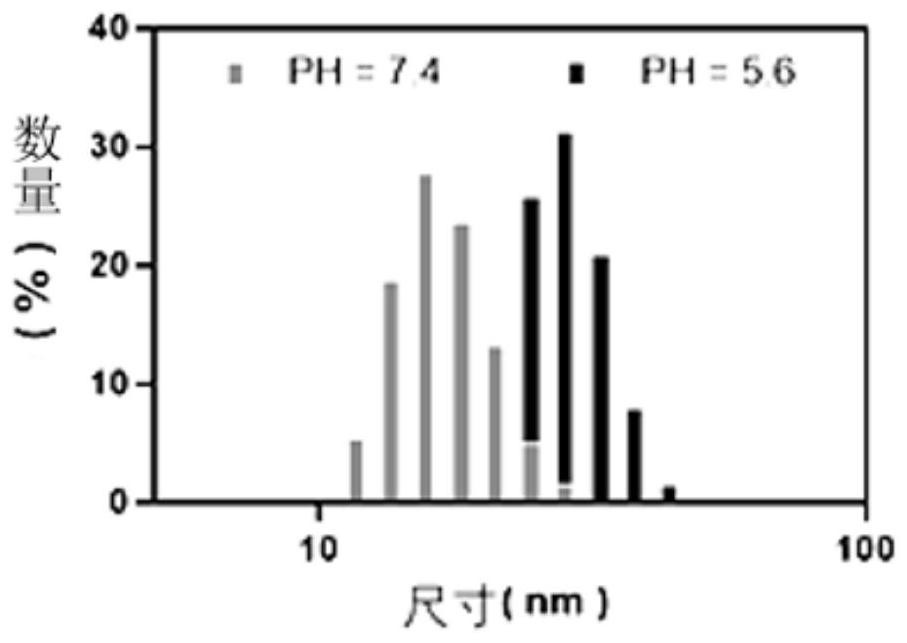
Stimuli-responsive nano material and application thereof in preparation of in-situ tumor vaccine
A technology of stimuli response and nanomaterials, applied in the direction of nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of genetic material changes, inability to effectively activate immune response, protein denaturation, etc., to enhance DC cell phagocytosis and enhance T cell Recruitment, effect of efficiency improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Temperature and pH stimulus-responsive nanomaterial APNPs and its preparation
[0071] This embodiment is a method for preparing temperature and pH stimulus-responsive nanomaterials, which specifically includes the following steps:
[0072] (1) Preparation of stimuli-responsive polymer carrier
[0073] Pluronic F127 (300mgmL) activated by 4-nitrophenyl chloroformate (4-NPC) -1 , 500mL) was dissolved in dichloromethane, under ultrasonic treatment (30% ultrasonic power, 3 minutes), was added dropwise to chitosan aqueous solution (15mgmL -1 , 5 mL, pH=10), after removing dichloromethane by rotary evaporation, the resulting solution was dialyzed overnight in deionized water with a dialysis tube (50 kDa), and then dialyzed in deionized water with a (1000 kDa) dialysis tube for 3 hours, Freeze-drying the prepared stimulus-responsive polymer carrier for further use;
[0074] (2) Surface functional modification of stimuli-responsive polymer carriers
[0075] Add ...
Embodiment 2
[0080] Example 2: Temperature and pH stimulus-responsive nanomaterials PNPs and their preparation
[0081] The preparation steps of APNPs in Example 1 were the same, except that astragalus polysaccharide (APS) was not added during encapsulation, so that temperature and pH stimulus-responsive nanomaterial PNPs were obtained.
Embodiment 3
[0082] Example 3: Other temperature and pH stimulus-responsive nanomaterials and their preparation
[0083] The difference between this embodiment and Example 1 is that the dichloromethane in the step (1) of Example 1 is replaced by other organic solvents such as styrene, perchlorethylene, trichloroethylene, ethylene glycol ether and triethanolamine Wait.
[0084] In this example, a temperature- and pH-stimulus-responsive nanomaterial for encapsulating astragalus polysaccharide and polyinosinic acid-polycytidylic acid was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com