Nucleic acid construct of epidermal growth factor, method of production and composition thereof
A technology of epidermal growth factor and nucleic acid constructs, applied in the field of biology, can solve problems such as inability to remove signal peptides or affinity tags
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
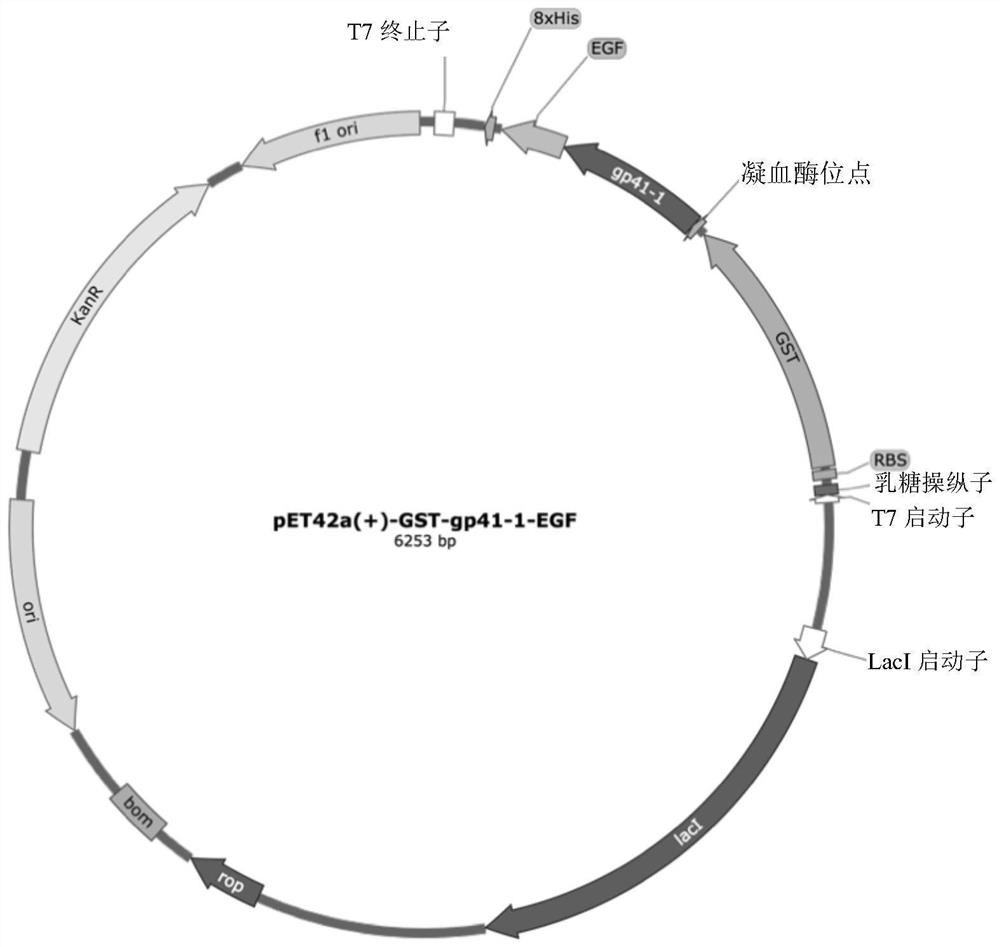
[0095] Embodiment 1: Construction of EGF expression vector
[0096] The expression construct pET42a(+)-GST-gp41-1-EGF was constructed as follows:
[0097] A synthetic DNA fragment (SEQ ID NO:5) encoding the 5' to 3' end sequence of NotI-stop codon-EGF-gp41-1-thrombin site-SpeI was synthesized by Thermo Fisher Scientific. The synthetic DNA fragment was amplified by PCR extension using the forward primer 5'-AAAAAGCGGCCGCTTAGCGCAGTTC-3' and the reverse primer 5'-AAAAAACTAGTCTGGTGCCACGCGGTAGTT-3'. The amplified PCR product was purified by PCR cleaning kit (Axygen AxyPrepClean-Up Kit) and digested with NotI and SpeI. The digested fragment was purified with 1% agarose and ligated into pET42a(+) digested with the same restriction enzymes.
[0098] The plasmid sequence of pET42a(+)-GST-gp41-1-EGF was confirmed by Sanger sequencing.
Embodiment 2
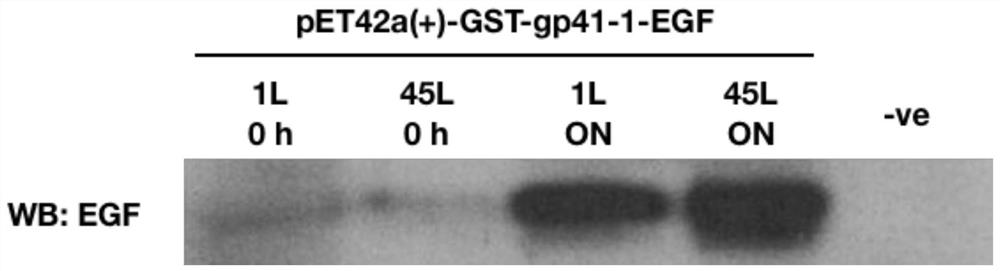
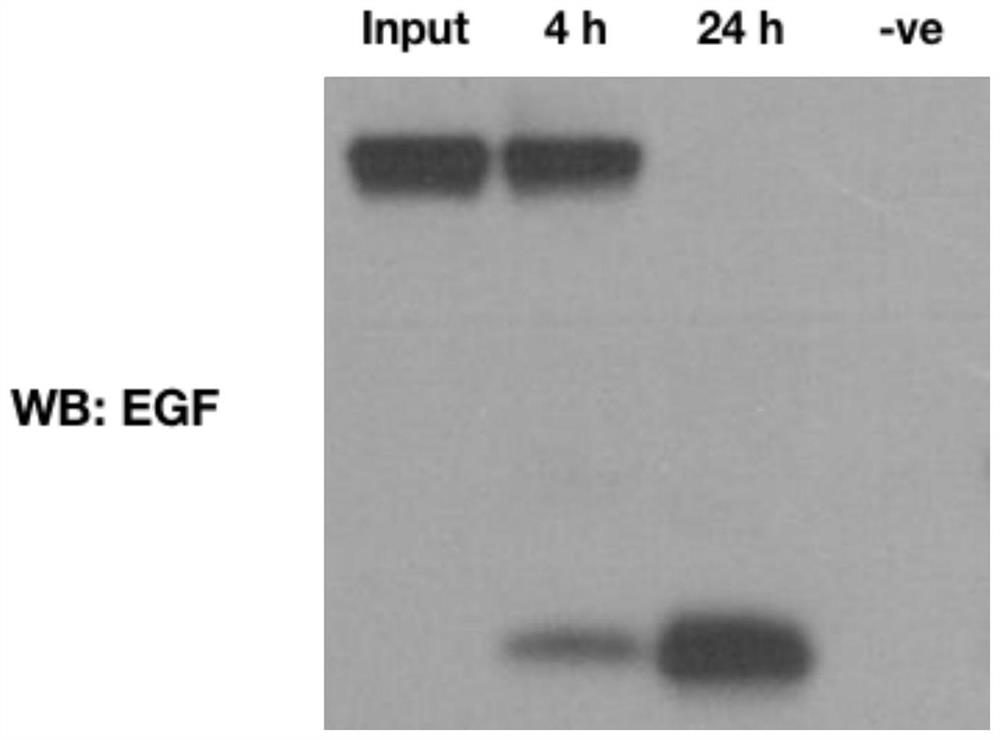
[0099] Embodiment 2: the expression of EGF fusion protein in shake flask
[0100] The plasmid pET42a(+)-GST-gp41-1-EGF was transformed into T7 Express (T7 Express). A single colony of transformants was supplemented with 40 μg·mL -1 Kanamycin was grown in 1 L of LB medium at 37°C (rotating at 250 rpm). When A 600 When the value reaches 0.5, reduce the growth temperature to 16 °C and add IPTG at a final concentration of 0.1 mM. Cultures were grown overnight. Collect 1 mL of the cell pellet and resuspend it in 200 μL of resuspension buffer (50 mM Tris-Cl, 200 mM EDTA, pH 8.0) supplemented with 1x PMSF, aprotinin, benzamide, and leupeptin, then Incubate for 5 minutes. Then at 37°C, with 120 μL lysozyme solution (1 mg·mL -1 ) to treat the mixture for 20 minutes. Then 80 μL of lysis buffer (10 mM EDTA, 10% Triton X-100 and 50 mM Tris-Cl, pH 8.0) was added. The tube containing the solution was gently inverted and then centrifuged at 14,800 rpm for 5 minutes. Cell lysate samp...
Embodiment 3
[0101] Embodiment 3: the expression of EGF fusion protein in fermentor
[0102] T7 expresses pET42a(+)-GST-gp41-1-EGF transformant, add 40μg·mL-1 Kanamycin was grown in 1 L of LB medium at 37°C (rotating at 250 rpm). When A 600 When the value reached 1, the whole culture was subcultured into a 50 liter fermenter containing 44 liters of LB medium. The culture was grown at 37 °C (rotation at 100 rpm, aeration ratio 1.5 vvm) until A 600 When the value reaches 0.5, reduce the growth temperature to 16 °C and add IPTG at a final concentration of 0.1 mM. 1M H in use 2 SO 4 The culture was grown overnight maintaining a pH of 7.0 with 1 M NaOH. Cell pellets were collected by serial centrifugation and washed twice with Buffer A (1x PBS and 1x PMSF, aprotinin, benzamide, and leupeptin) for long-term storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com