Azo-structure disperse dye for dyeing polypropylene fibers and preparation method and dyeing process of azo-structure disperse dye
A polypropylene fiber and disperse dye technology, applied in the direction of azo dyes, monoazo dyes, disazo dyes, etc., can solve the problems of poor color fastness and low dye uptake, and achieve strong permeability and easy dyeing Dyeing, the effect of small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
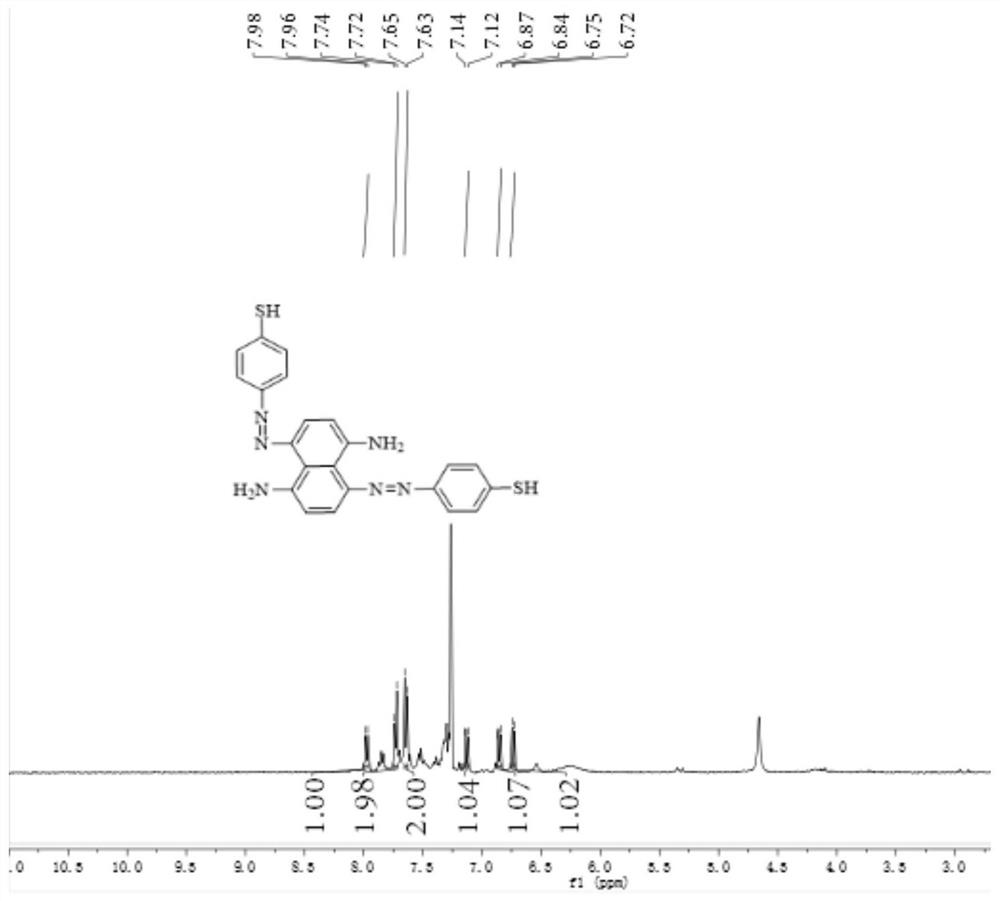
[0056] Weigh 0.248g (2mmol) of p-aminothiophenol and dissolve it in a dilute hydrochloric acid solution. The dilute hydrochloric acid solution is formed by mixing 1440 μL of concentrated hydrochloric acid and 15 mL of distilled water. The above solution is placed in an ice bath and stirred with a rotor. Dissolve 0.15g (2.17mmol) of sodium nitrite in 8mL of distilled water, quickly add the sodium nitrite aqueous solution to the mixed solution of p-aminothiophenol and hydrochloric acid solution, the solution changes from colorless to brownish-yellow clear solution, in ice bath The reaction was carried out under the same conditions for 1 min, and the reaction end was detected using Ehrlich reagent and starch potassium iodide test paper to obtain a diazotized product, and sulfamic acid was used to remove excess nitrous acid.
[0057] In addition, 0.158g (1mmol) of 1,5-diaminonaphthalene was weighed and dissolved in 3mL of acetone solution, and cooled with ice water; the above-synth...
Embodiment 2
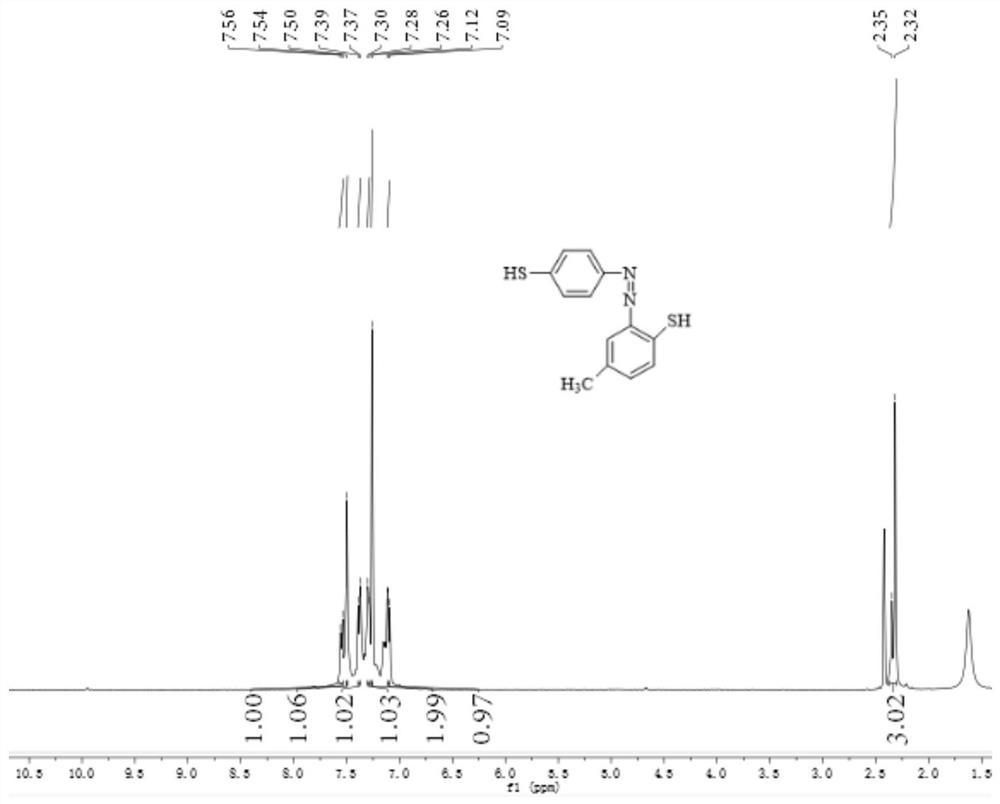
[0066] Weigh 0.124g (1mmol) of p-aminothiophenol and dissolve it in a dilute hydrochloric acid solution. The dilute hydrochloric acid solution is formed by mixing 720 μL of concentrated hydrochloric acid and 10 mL of distilled water. The above solution is placed in an ice bath and stirred with a rotor. Dissolve 0.076g (1.1mmol) of sodium nitrite in 4mL of distilled water, quickly add the sodium nitrite aqueous solution to the mixed solution of p-aminothiophenol and dilute hydrochloric acid solution, the solution changes from colorless to brownish-yellow clear solution. The reaction was carried out in a bath for 1 min, and the reaction end was detected using Ehrlich reagent and starch potassium iodide test paper to obtain a diazotized product, and sulfamic acid was used to remove excess nitrous acid.
[0067] In addition, 0.124g (1mmol) of p-methylthiophenol was weighed and dissolved in 3mL of acetone solution, and cooled with ice water; the above-mentioned synthetic diazotized ...
Embodiment 3
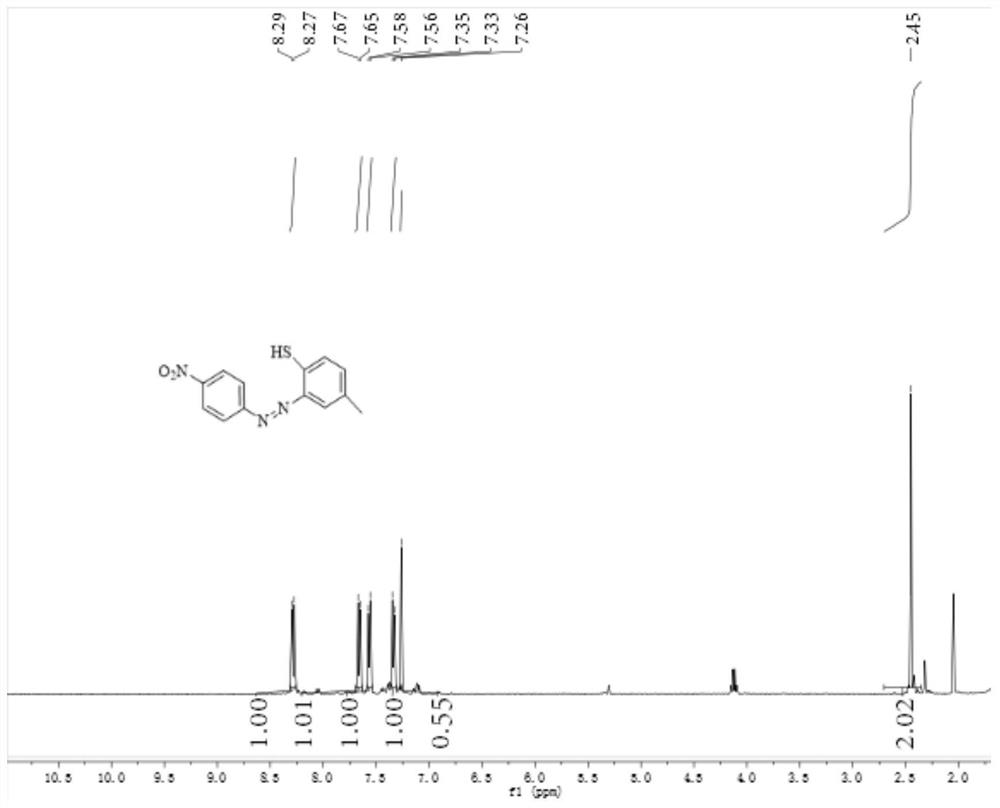
[0076] Weigh 0.138g (1mmol) of p-nitroaniline, dissolve it in a dilute hydrochloric acid solution, and this dilute hydrochloric acid solution is formed by mixing 720 μL of concentrated hydrochloric acid and 10 mL of distilled water. The above solution is placed in an ice bath and stirred with a rotor. 0.076g (1.1mmol) of sodium nitrite was dissolved in 4mL of distilled water, and the sodium nitrite aqueous solution was quickly added to the mixed solution of p-nitroaniline and dilute hydrochloric acid solution. The reaction was carried out under the conditions for 1 min, and the reaction end was detected using Ehrlich reagent and starch potassium iodide test paper to obtain a diazotized product, and sulfamic acid was used to remove excess nitrous acid.
[0077] In addition, 0.124g (1mmol) of p-methylthiophenol was weighed and dissolved in 3mL of acetone solution, and cooled with ice water; the above-mentioned synthetic diazotized product was added dropwise to it to carry out cou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color fastness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com