Application of cationic polymer in preparation of drugs for removing intestinal microbial toxins and treating tumors
A technology of intestinal microorganisms and cationic polymers, which can be used in the field of medicine to solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
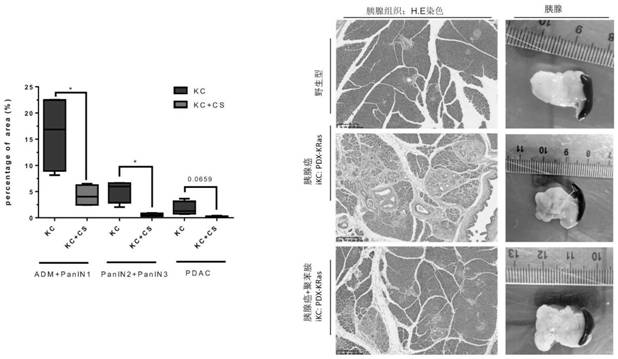
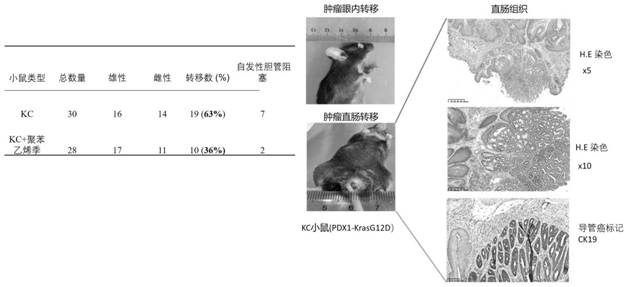
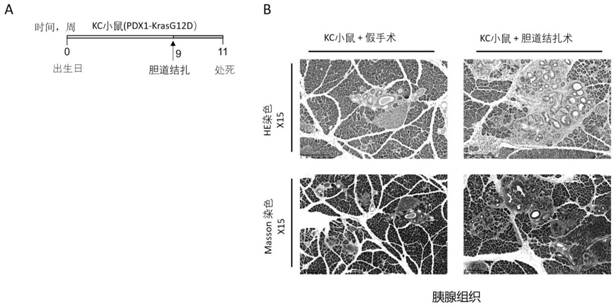
[0092] The preparation and clinical application of the high-molecular cationic polymer of the present invention include treating, alleviating and preventing pancreatic cancer and other cancers, including their various complications. As an example of implementation, it includes amine polymer; its preferred practical application is polystyrene quaternary ammonium salt, cholestyramine (cholestyramine, referred to as "polyaniline"). Repeating units derived from the polymerization of linked monomers, such as polystyrene quaternary ammonium salt ([4-[3-(4-ethylphenyl)butyl]phenyl]-trimethylazanium), whose molecular formula is C 21 h 30 N+, including its various derivative structures, such as modified polyallylamine, copolymerof diethylenetriamine, (C 4 h 10 N 3 ) m (C 3 h 6 O) n ). Its molecular mass (molecular mass) exceeds 1x10 6 g / mol. The general formula of its molecular structure is as follows:
[0093]
[0094] The cationic resin can be taken orally alone, or adde...
Embodiment 2
[0096] The preparation and clinical application of the high-molecular cationic polymer of the present invention include treating, alleviating and preventing pancreatic cancer and other cancers, including the complications of these cancers. As an example of implementation, amine polymers are included. As the preferred application, Colestipol (Colestipol, hydrochloride, Epichlorhydrin-tetraethylenepentamine polymer, CAS No.37296-80-3; SDS CAS No:37296-80-3; Molecular Weight: (225.765) n; Molecular Formula: C 8 h 24 CLN5), or similar cation-rich polymers.
[0097] Its molecular mass (molecular mass) exceeds 1x10 6 g / mol. The general formula of its molecular structure is as follows:
[0098] Colestipol molecular formula: (C 4 h 10 N 3 ) m (C 3 h 6 O) n .
[0099] Its molecular structure general formula is as follows:
[0100]
[0101] Therefore, the high-molecular-weight cationic polymer can be used for the treatment, prevention of said diseases, and the alleviatio...
Embodiment 3
[0104] Colesevelam is another high-molecular cationic polymer, and its bile acid binding capacity is 7 times that of cholestyramine. Based on the similarity of its mechanism, we speculate that oral administration of colesevelam can also reduce the endotoxin produced by intestinal cells and the endotoxin in the blood, thereby reducing the systemic inflammation of the body, restoring insulin sensitivity, reducing fatty liver, and promoting Self-ablation of NASH and cirrhosis. The daily dosage can be viewed as 3.75g, which can be administered in powder or tablet form. The molecular formula of colesevelam is C 31 h 67 Cl 3 N 4 o ; International Union of Pure and Applied Chemistry (IUPAC) named: 2-(chloromethyl)oxirane; prop-2-en-1-amine; N-prop-2-enyldecan-1-amine; trimethyl-[6-(prop -2-enylamino)hexyl]azanium; chloride; hydrochloride.
[0105]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com