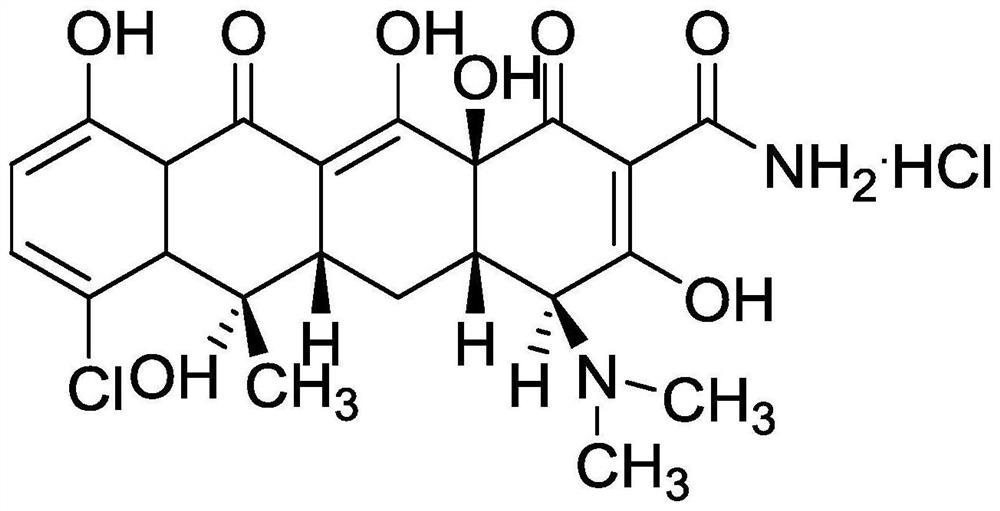
Preparation method of chlortetracycline hydrochloride
The technology of chlortetracycline hydrochloride and chlortetracycline, which is applied in the field of preparation of chlortetracycline hydrochloride, can solve the problems of high extraction cost and large pollution of chlortetracycline hydrochloride, and achieves a green and environment-friendly production process, is easy to operate, and has a simple crystallization process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of aureomycin hydrochloride, comprising the steps of:
[0031] 1) Cool 20L of chlortetracycline fermentation broth, lower the temperature to 20°C, add oxalic acid at a volume ratio of 2.0% (g / mL), adjust the pH to 1.1 with concentrated hydrochloric acid, and then use a weight-volume ratio of 0.15% (g / mL) Add yellow blood salt (sodium salt) and zinc sulfate to the above fermentation broth, wherein yellow blood salt and zinc sulfate are stirred for 25 minutes according to the weight ratio of 1:1, and then add flocculant according to the volume ratio of 0.5% (mL / mL) No. 1, stirred for 15 minutes, and the stirred fermentation broth was filtered through a plate frame to obtain 10L of acidified filtrate;
[0032] The No. 1 flocculant is selected from polyacrylamide polymers;
[0033] 2) Collect the 10L chlortetracycline acidification filtrate obtained in the previous step (using spectrophotometry to measure its chemical potency as 12300u / ml) and put it i...
Embodiment 2
[0045] A preparation method of aureomycin hydrochloride, comprising the steps of:
[0046] 1) Cool 300L of chlortetracycline fermentation broth, lower the temperature to 15°C, add oxalic acid at a volume ratio of 1.2% (g / mL), adjust the pH to 1.4 with concentrated hydrochloric acid, and then use a mass-volume ratio of 0.1% (g / mL) Add yellow blood salt (potassium salt) and zinc sulfate to the above fermentation broth, wherein yellow blood salt and zinc sulfate are stirred for 20 minutes according to the weight ratio of 1:1, and then add flocculant according to the volume ratio of 1.0% (mL / mL) No. 1, stirred for 10 minutes, and the stirred fermented liquid was filtered through a plate frame to obtain 150 L of acidified filtrate;
[0047] The No. 1 flocculant is selected from polyacrylamide polymers;
[0048] 2) Collect the 150L chlortetracycline acidification filtrate obtained in the previous step (using spectrophotometry to measure its chemical potency as 10000u / ml) and put it...
Embodiment 3
[0058] A preparation method of aureomycin hydrochloride, comprising the steps of:
[0059] 1) Cool 20L of chlortetracycline fermentation broth, lower the temperature to 18°C, add oxalic acid at a volume ratio of 1.5% (g / mL), adjust the pH to 1.2 with concentrated hydrochloric acid, and then use a weight-volume ratio of 0.2% (g / mL) Add yellow blood salt (sodium salt) and zinc sulfate to the above fermentation broth, wherein yellow blood salt and zinc sulfate are stirred for 30 minutes according to the weight ratio of 1:1, and then add flocculant according to the volume ratio of 0.8% (v / v) No. 1, stirred for 20 minutes, and the stirred fermented liquid was filtered through a plate frame to obtain 10 L of acidified filtrate;
[0060] The No. 1 flocculant is selected from polyacrylamide polymers;
[0061] 2) Collect the 10L chlortetracycline acidification filtrate obtained in the above step (using spectrophotometry to measure its chemical potency as 12000u / ml) and put it in the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com