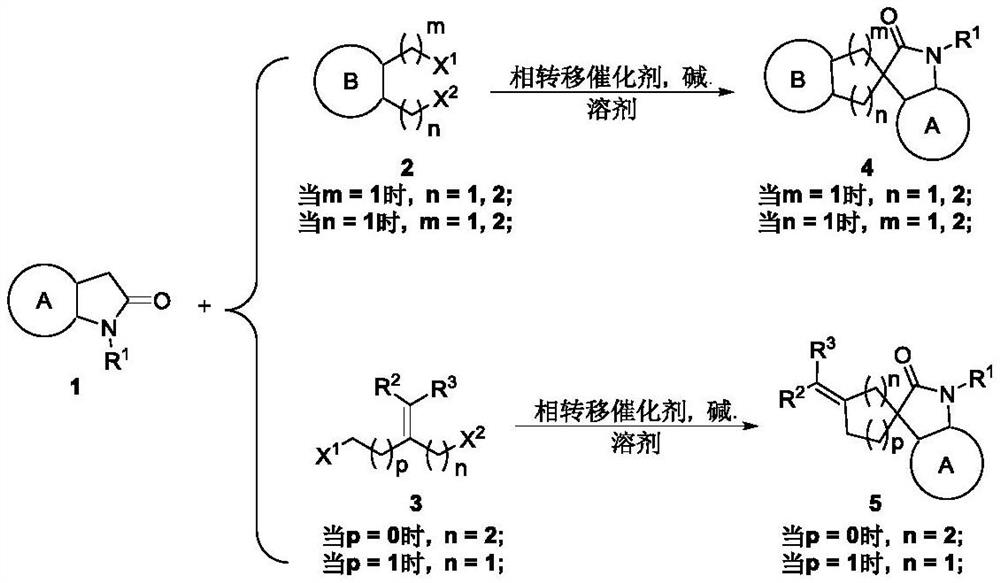
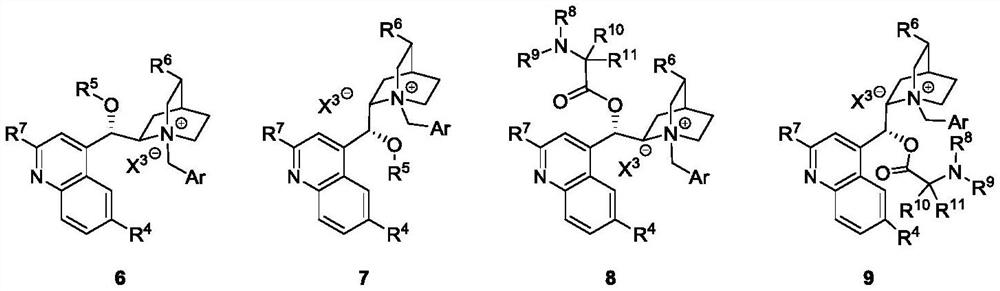
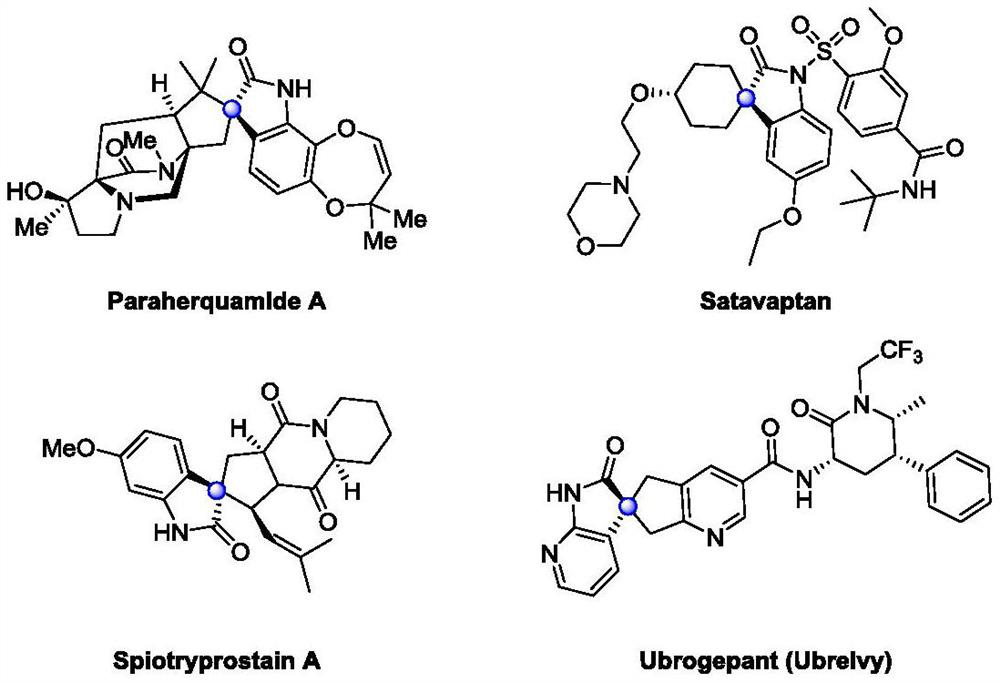
Catalytic asymmetric synthesis method and application of chiral oxindole spiro analogue
A technology for the synthesis of oxindole spiro and its synthesis method, which is applied in the synthesis of key intermediates of the drug ubrogepant, and the preparation of chiral five-membered and six-membered carbocyclic oxindole spiro compounds and analogues, which can solve the problem of inability to realize spiro rings Unified structural compounds, general synthesis, difficulty in preparation of chiral ligands, unfavorable large-scale production and other problems, to achieve high industrial application value, save preparation costs, and apply to a wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of compound 5a
[0059]
[0060] Add indolinone substrate 1a (56mg, 0.24mmol, 1.2equiv), diiodide compound 3a (64mg, 0.2mmol, 1.0equiv) and phase transfer catalyst 8a (15mg, 0.02mmol, 0.1equiv) sequentially into a 10mL reaction test tube , and then added toluene / dichloromethane (4 mL / 0.8 mL) to dissolve. At -60°C, 80% CsOH aqueous solution (65 μL, 1.0 mmol, 5.0 equiv) was added into the reaction tube, and then reacted at this temperature for about 34 hours. After the reaction was complete, 2 mL of saturated ammonium chloride solution was added, and then the aqueous solution was extracted 3 times with 10 mL of ethyl acetate. Combined extracts, anhydrous Na 2 SO 4After drying, filtering and concentrating, the residue was separated and purified by silica gel column chromatography to obtain chiral compound 5a (40 mg) with a yield of 67%.
[0061] Pale yellow oil. 1 H NMR (400MHz, CDCl 3 )δ7.84(d, J=8.2Hz, 1H), 7.29(d, J=8.0Hz, 1H), 7.22(d, J=7.4Hz, 1H),...
Embodiment 2
[0064] Preparation of compound 5b
[0065]
[0066] Add indolinone substrate 1b (59mg, 0.24mmol, 1.2equiv), diiodide compound 3a (64mg, 0.2mmol, 1.0equiv) and phase transfer catalyst 8a (15mg, 0.02mmol, 0.1equiv) successively in a 10mL reaction test tube , and then added toluene / dichloromethane (4 mL / 0.8 mL) to dissolve. At -60°C, 80% CsOH aqueous solution (65 μL, 1.0 mmol, 5.0 equiv) was added into the reaction tube, and then reacted at this temperature for about 39 hours. After the reaction was complete, 2 mL of saturated ammonium chloride solution was added, and then the aqueous solution was extracted 3 times with 10 mL of ethyl acetate. Combined extracts, anhydrous Na 2 SO 4 After drying, filtering and concentrating, the residue was separated and purified by silica gel column chromatography to obtain chiral compound 5b (44 mg) with a yield of 70%.
[0067] Pale yellow oil. 1 H NMR (400MHz, CDCl 3 )δ7.72(s,1H),7.09(d,J=7.6Hz,1H), 6.95(d,J=7.6Hz,1H),5.04(s,1H),5.01(...
Embodiment 3
[0070] Preparation of compound 5c
[0071]
[0072] Add indolinone substrate 1c (63mg, 0.24mmol, 1.2equiv), diiodide compound 3a (64mg, 0.2mmol, 1.0equiv), phase transfer catalyst 8a (15mg, 0.02mmol, 0.1equiv) in sequence in a 10mL reaction test tube , and then added toluene / dichloromethane (4 mL / 0.8 mL) to dissolve. At -60°C, 80% CsOH aqueous solution (65 μL, 1.0 mmol, 5.0 equiv) was added into the reaction tube, and then reacted at this temperature for about 36 hours. After the reaction was complete, 2 mL of saturated ammonium chloride solution was added, and then the aqueous solution was extracted 3 times with 10 mL of ethyl acetate. Combined extracts, anhydrous Na 2 SO 4 After drying, filtering and concentrating, the residue was separated and purified by silica gel column chromatography to obtain chiral compound 5c (43 mg) with a yield of 65%.
[0073] white solid. 1 H NMR (400MHz, CDCl 3 )δ7.76(d,J=9.7Hz,1H),6.84–6.72(m,2H),5.05(s,1H),5.02(s,1H),3.79(s,3H),2.95(d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com