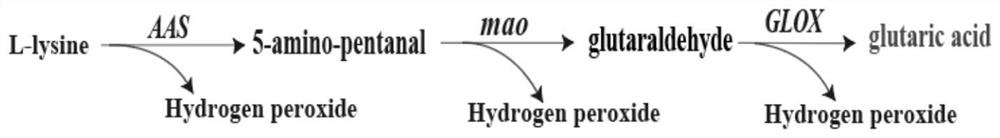
Recombinant escherichia coli capable of efficiently producing glutaric acid as well as construction method and application of recombinant escherichia coli
A technology for recombining Escherichia coli and Escherichia coli, which is applied in the field of metabolic engineering, can solve the problems of too many pathway enzymes and complex pathways, and achieve the effect of efficient production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Construction of recombinant plasmid pETM6R1-AAS-Mao
[0041] Amplifying the coding gene of the aromatic aldehyde synthase AAS whose amino acid sequence is shown in SEQ ID NO.1 and the coding gene of amine oxidase Mao whose amino acid sequence is shown in SEQ ID NO.2, to obtain the aromatic aldehyde synthase AAS The DNA fragment of the coding gene of amine oxidase Mao and the DNA fragment of the coding gene containing amine oxidase Mao, select the restriction endonuclease XhoI, digest the plasmid pETM6R1 overnight to obtain the linearized vector and expose the cohesive end, purify and recover The DNA fragment containing the coding gene of aromatic aldehyde synthase AAS and the DNA fragment containing the coding gene of amine oxidase Mao were connected to the vector pETM6R1 by enzyme digestion and ligation, and the recombinant plasmid pETM6R1-AAS-Mao was constructed, and the obtained recombinant Plasmid pETM6R1-AAS-Mao was transformed into E.coli JM109 competen...
Embodiment 2
[0042] Embodiment 2: Construction of recombinant plasmid pET28a-Glox
[0043] Amplify the coding gene of aldehyde dehydrogenase Glox whose amino acid sequence is shown in SEQ ID NO.3 to obtain a DNA fragment containing the coding gene of aldehyde dehydrogenase Glox, and use restriction endonucleases BamHI and XhoI at 37°C The vector pET28a was double-digested for 3 hours to obtain a linearized vector and expose the cohesive ends. The Glox-encoding gene after digestion and gel recovery was ligated with the plasmid pET28a at 16°C for 10 hours with T4 ligase to construct the recombinant plasmid pET28a-Glox. The constructed recombinant plasmid pET28a-Glox was transformed into E.coli JM109 competent cells by chemical transformation method, cultured on LB plates containing kanamycin for 12 hours, and the colonies grown on the plates were verified by PCR. Positive transformants were selected and inoculated in LB medium, cultured at 37°C for 12 hours, and plasmids were extracted. Aft...
Embodiment 3
[0044] Example 3: Construction and expression of double-plasmid recombinant Escherichia coli
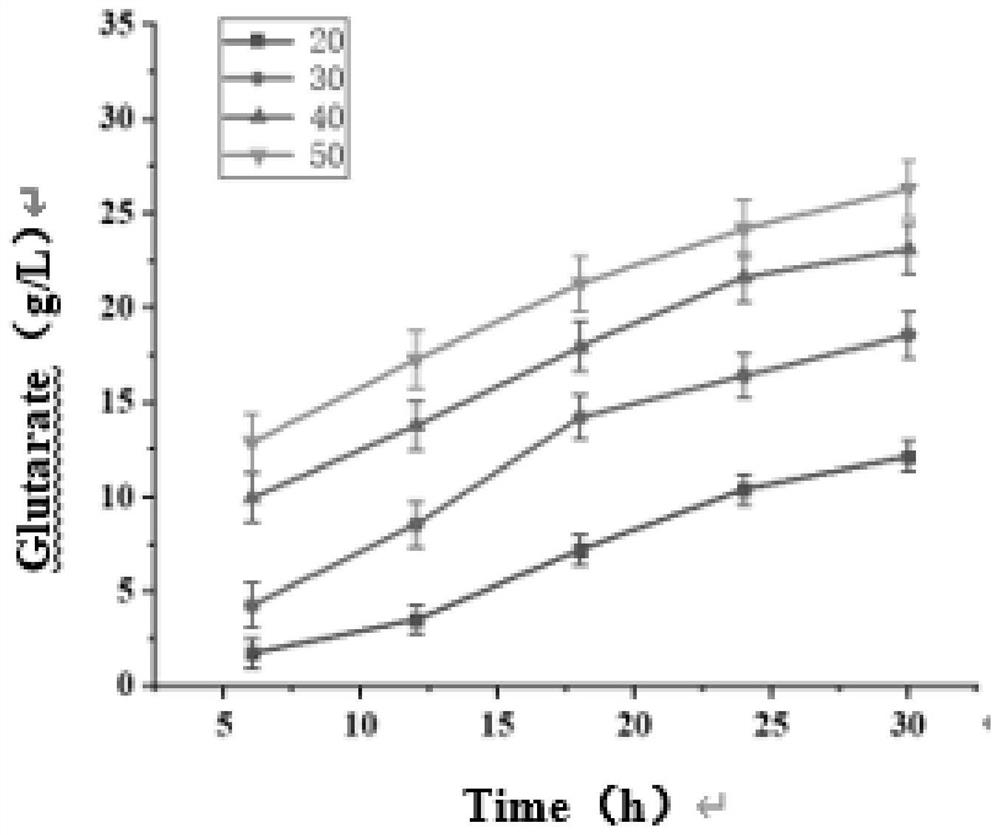
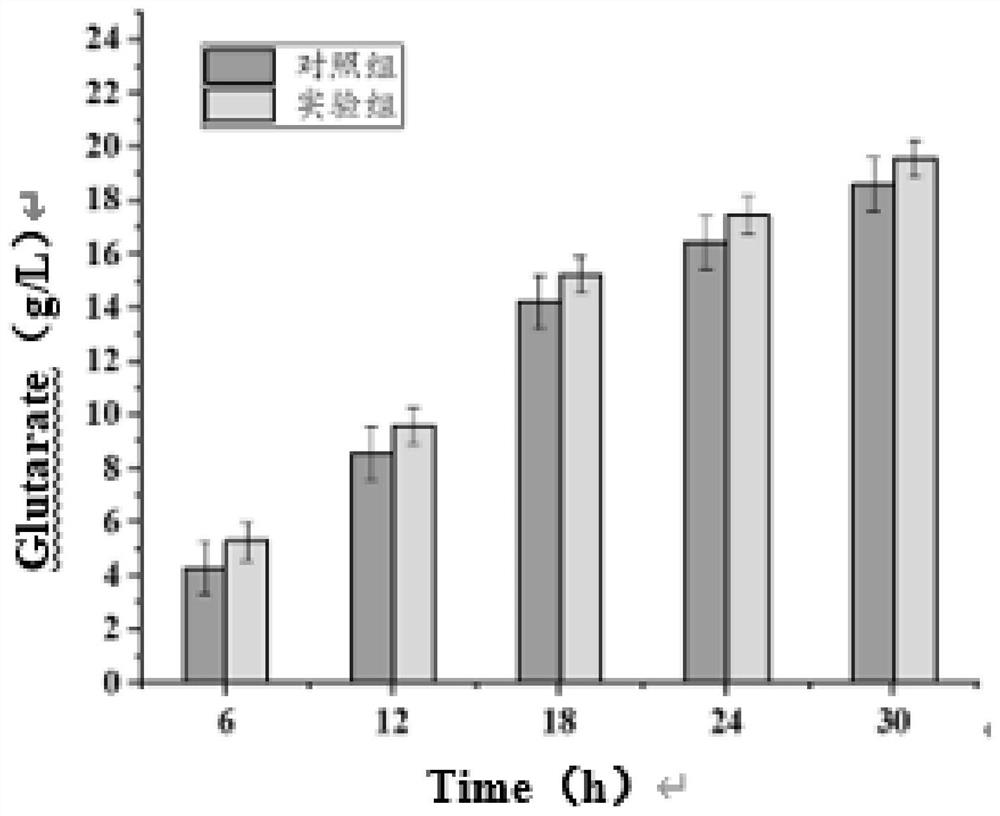
[0045] The recombinant plasmid pETM6R1-AAS-Mao constructed in Example 1 and the recombinant plasmid pET28a-Glox constructed in Example 2 were simultaneously transformed into E.coli MG1655 (DE3) competent cells by chemical transformation method, in the presence of ampicillin and cardamic Cultivate for 12 hours on LB plates with two kinds of resistance to namycin, and inoculate the single colony grown on the plate in the seed medium containing ampicillin and kanamycin resistance, and culture overnight at 37°C and 200rpm. Transfer to 100mL fermentation medium containing ampicillin and kanamycin resistance with 2% (v / v) inoculum size, cultivate to OD at 37°C and 200rpm 600 0.6-0.8, adding IPTG with a final concentration of 1mM for induction, and after induction at 25°C and 200rpm for 12h, the bacteria were collected by centrifugation for whole cell transformation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com