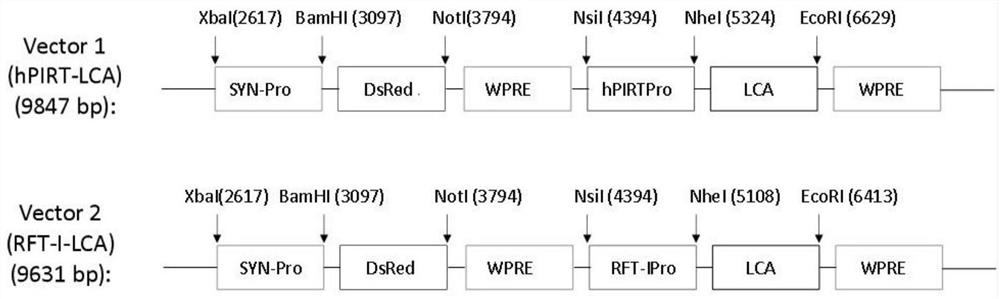
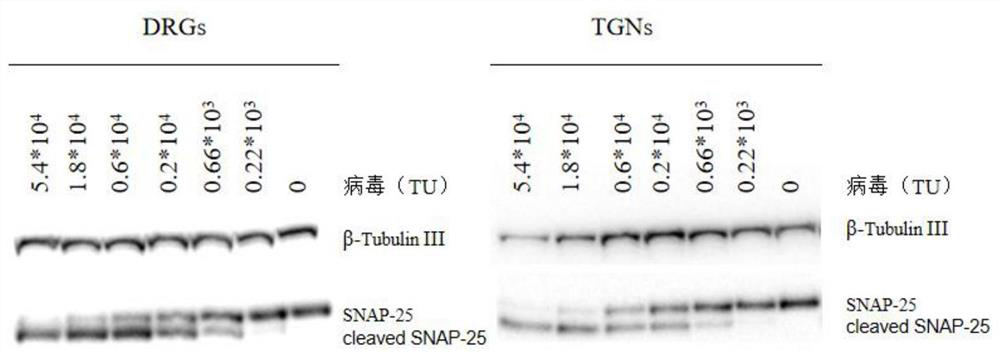
Expression unit, recombinant lentivirus expression vector, recombinant lentivirus and preparation method and application of recombinant lentivirus
A technology of recombinant lentivirus and expression vector, applied in the field of recombinant lentivirus and its preparation, can solve the problems of long course of disease, complex etiology, and easy emergence of drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a method for preparing the above-mentioned recombinant lentivirus, which specifically includes the following steps:
[0043] It is obtained by co-transfecting the above-mentioned recombinant lentiviral expression vector with the lentiviral envelope vector and the packaging vector into a cell line.
[0044] In the present invention, the lentiviral envelope vector is preferably pMD2.G; the packaging vector is preferably psPAX2; and the cell line is preferably 293T cells. The present invention has no special limitation on the transfection method, and conventional transfection methods in the art can be used.
[0045] The present invention also provides the application of the above expression unit, recombinant lentiviral expression vector or recombinant lentivirus in the preparation of drugs for treating chronic pain.
Embodiment 1
[0050] 1. Construction of recombinant lentiviral expression vector 1
[0051] (1) Using the Ensembl tool to analyze the human PIRT gene, artificially synthesize the sequence SEQ ID NO.1 of the PIRT gene promoter, the 5' end restriction endonuclease Nsi I and the 3' end NheI;
[0052] (2) Digest the plasmid containing the artificially synthesized PIRT gene promoter element in step (1) with NsiI and NheI, and recover about 930 bp by gel electrophoresis to obtain the hPIRT promoter fragment.
[0053] (3) Ligate the hPIRT promoter fragment with T4 DNA ligase and the large fragment of the SYN-DsRed-SYN-GFP plasmid that was double-digested with Nsi I and Nhe I and recovered by gel electrophoresis, transform TOP 10 competent bacteria, and pick Bacterial culture was performed on a single colony and the plasmid was extracted for Nsi I and Nhe I digestion and sequencing identification to obtain the SYN-DsRed-hPIRT-GFP vector;
[0054] (4) In vitro synthesis sequence such as the botulin...
Embodiment 2
[0059] Example 2 Identification and Extraction of Recombinant Lentiviral Expression Vector
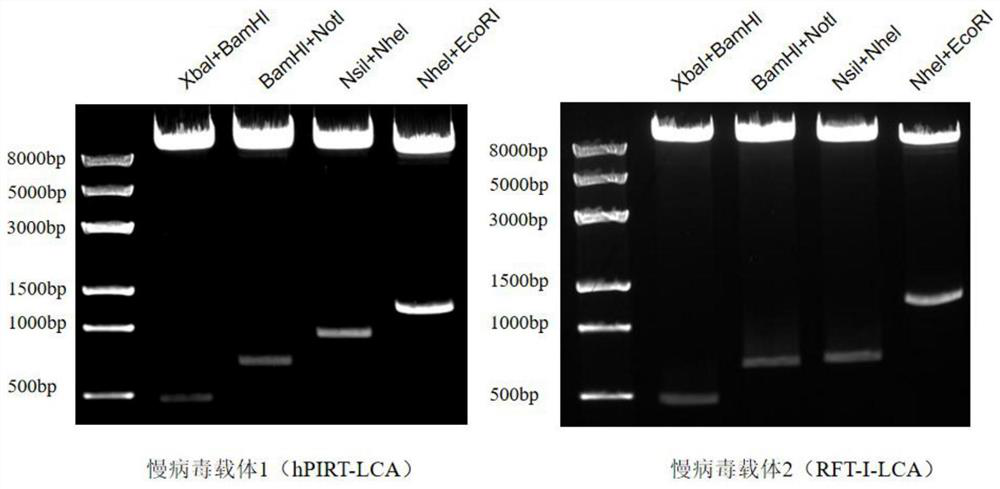
[0060] 1. Group double enzyme digestion to verify the correctness of the recombinant lentiviral expression vector construction
[0061] Take a 200 μL centrifuge tube, add each of the reagents in Table 1 to the centrifuge tube in turn, gently pipette to mix, and place it in a 37°C water bath for 3 hours. After 3 hours, add 10 μL 6×Loading Buffer to each centrifuge tube to stop the enzyme digestion and mix gently by pipetting. A 0.7% agarose nucleic acid gel was prepared and loaded for agarose gel electrophoresis. After electrophoresis, the nucleic acid gel was placed in G-BOX to take pictures, and the attached figure 2 .
[0062] Table 1 Add reagents
[0063] Reagent Volume (μL) Recombinant lentiviral expression vector DNA (1 μg / μL) 1 10×NEB Buffer 5 h 2 o
42 Enzyme 1 1 Enzyme 2 1 Total 50
[0064] Among them, different promo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com