Process and device for preparing lithium carbonate from carbonic acid type brine
A technology of lithium carbonate and carbonic acid type, applied in lithium carbonate;/acid carbonate, improvement of process efficiency, membrane technology, etc., can solve the problem of long lithium extraction cycle, low product purity, low lithium yield, etc. problems, achieve the effect of shortening the lithium extraction cycle, improving the lithium recovery rate, and no environmental hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
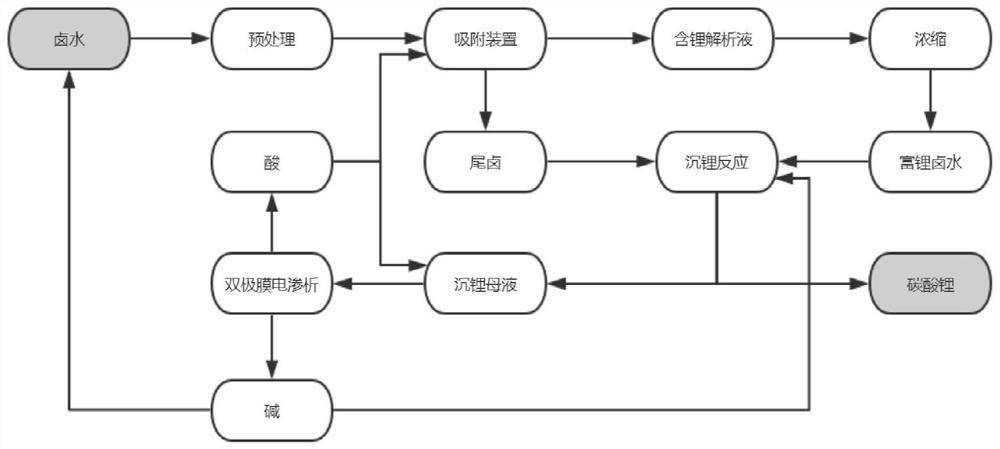
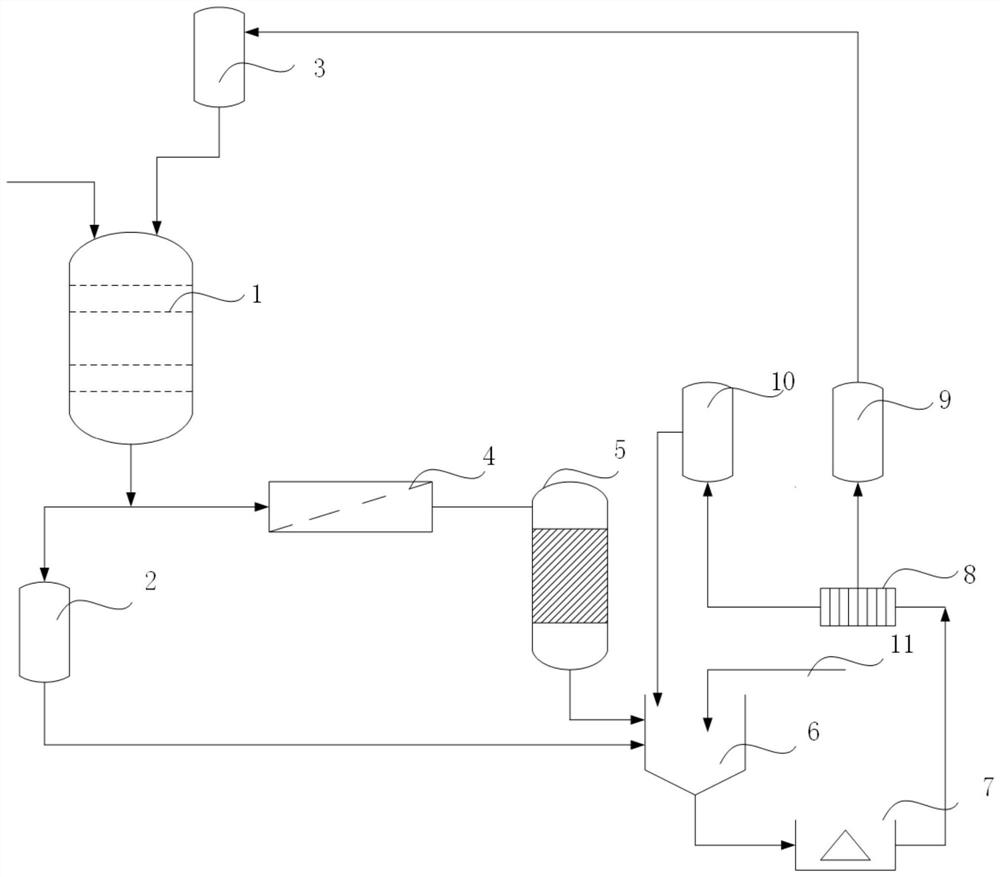
[0065] This embodiment provides a process for preparing lithium carbonate from a carbonated salt lake brine. The brine used is a carbonate-type brine from a certain salt lake in Tibet. The main ions in the original brine are lithium ions, sodium ions, magnesium ions, boron elements and carbonate radicals. The ion concentrations are 0.30g / L, 44.0g / L, 0.08g / L, 0.6g / L and 18.9g / L respectively, combining figure 1 Shown is the flow process of this embodiment, the method of this embodiment includes the following steps:
[0066] The above-mentioned carbonated salt lake brine is sent to a pretreatment multi-media filter to filter and remove some mechanical impurities such as sediment to obtain pretreated brine.
[0067] The brine after the pretreatment is sent to a device equipped with a titanium-based adsorbent. After the adsorption is completed, 0.3mol / L sulfuric acid is used for analysis and desorption to obtain the adsorption tail brine and lithium-containing analysis solution. Th...
Embodiment 2
[0072] This embodiment provides a process for preparing lithium carbonate from a carbonated salt lake brine. The brine used is a carbonate-type brine from a certain salt lake in Tibet. The main ions in the original brine are lithium ions, sodium ions, magnesium ions, boron elements and carbonate radicals. The ion concentrations are 0.81g / L, 142g / L, 0.1g / L, 3g / L and 28.9g / L respectively, combining figure 1 Shown is the flow process of this embodiment, the method of this embodiment includes the following steps:
[0073] The above-mentioned carbonated salt lake brine is sent to a pretreatment multi-media filter to filter and remove some mechanical impurities such as sediment to obtain pretreated brine.
[0074] The brine after pretreatment is sent to a device equipped with a titanium-based adsorbent. After the adsorption is completed, 0.4mol / L sulfuric acid is used for analysis and desorption to obtain the adsorption tail brine and lithium-containing analysis solution. The concen...
Embodiment 3
[0079] The brine used in this embodiment is a certain salt lake lithium sinking mother liquor, the concentration of main ion lithium ion, sodium ion and carbonate ion in this mother liquor is respectively 1.81g / L, 32.5g / L and 18.9g / L, combining figure 1 Shown is the flow process of the present embodiment, and other conditions are identical with embodiment 2, obtain lithium carbonate product purity and reach 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com