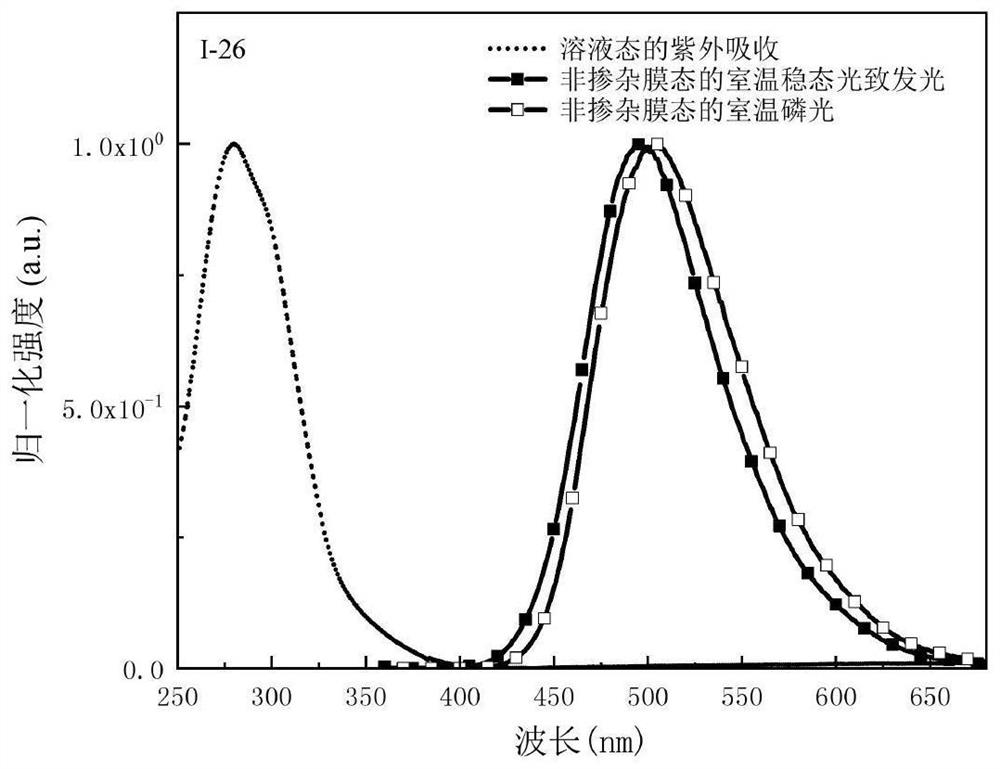
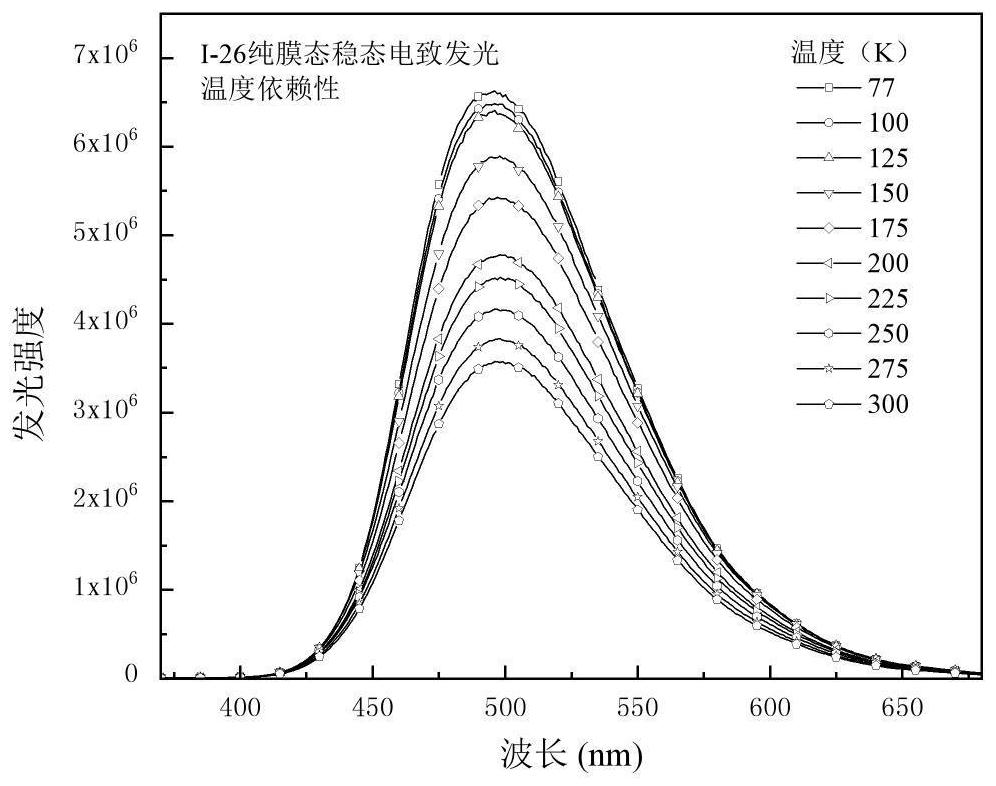
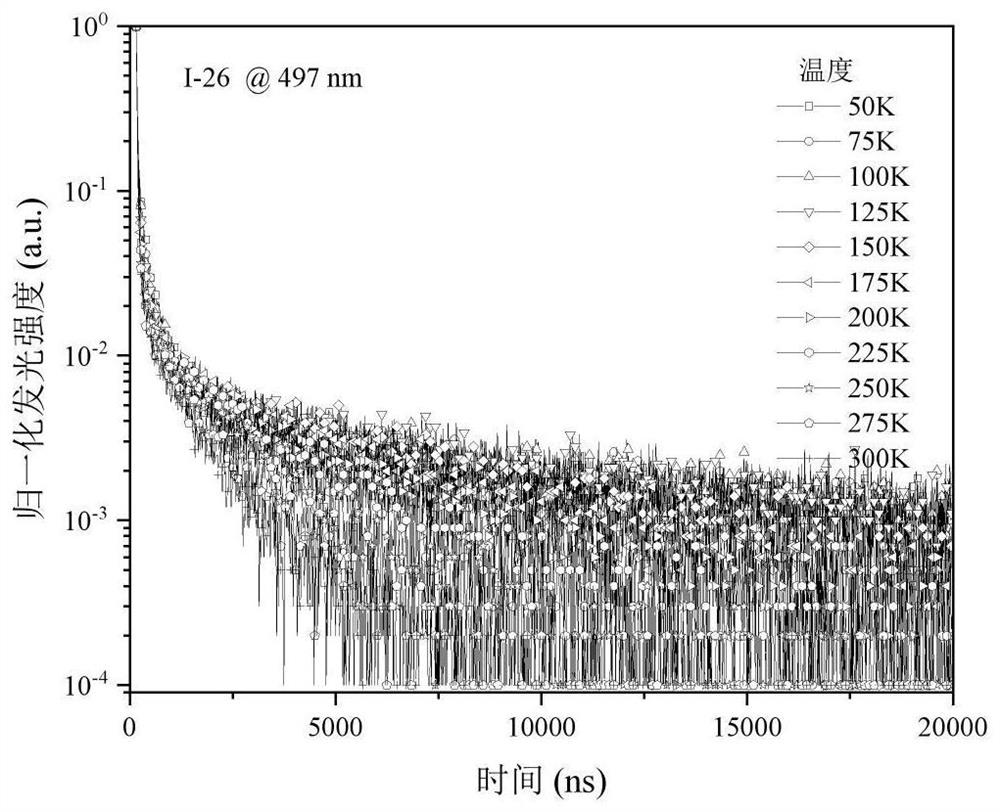
Organic room-temperature electrophosphorescent material, preparation method and organic electroluminescent diode thereof
An electrophosphorescence and electroluminescence technology, which is applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as restricting the application of OLED devices, and achieve the effect of enhancing the spin-orbit coupling effect and suppressing non-radiative attenuation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] The present invention provides a method for preparing an organic room temperature electrophosphorescent material described in the above technical solution, comprising the following steps:
[0085] In a protective atmosphere, the intermediate D-XH, the intermediate F-A, an organic solvent, a dehydrating agent and a basic catalyst are subjected to a nucleophilic substitution reaction to obtain the organic room-temperature electrophosphorescent material having the structure of formula I.
[0086] In the present invention, -XH is connected to the benzene ring in the D structure.
[0087] In the present invention, the D-XH is specifically the intermediate D-OH or the intermediate D-SH.
[0088] The present invention has no special requirements on the source of the intermediate D-XH, and commercially available or self-made products can be used. In the present invention, when the intermediate D-OH or the intermediate D-SH is preferably a self-made product, the present inventi...
Embodiment 1
[0172] Add M1 (1g, 3.32mmol) and potassium carbonate (0.55g, 3.98mmol) into a three-necked reaction flask, and under the protection of argon, add 5mL of N-methylpyrrolidone and 5mL of toluene. Carry out at 140°C, and reflux with a condenser connected to a water separator. After 2h, the reaction was cooled to room temperature. Add 5mL of M2 (0.86g, 2.59mmol) dissolved in N-methylpyrrolidone, raise the temperature to 190°C for affinity substitution reaction for 18h, and detect the reaction by thin-layer chromatography. After the reaction was completed, it was washed three times with deionized water, and the organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, and concentrated. Silica gel column chromatography, eluting with a mixed solvent of petroleum ether:dichloromethane with a volume ratio of 5:1, and drying to obtain a white solid organic room temperature electrophosphorescent material with the structural formula I-26, 1.554 g, and a yield ...
Embodiment 2
[0178] Add M3 (0.36g, 1.53mmol) and potassium carbonate (0.25g, 1.83mmol) into a three-necked reaction flask, and under the protection of argon, add 5mL of N-methylpyrrolidone and 5mL of toluene. Carry out at 140°C, and reflux with a condenser connected to a water separator. After 2h, the reaction was cooled to room temperature. Add 5mL of M2 (0.396g, 1.19mmol) dissolved in N-methylpyrrolidone, heat up to 190°C, and detect the reaction by thin-layer chromatography. After the reaction, the filtrate was washed with deionized water, the organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, and concentrated. Silica gel column chromatography, eluting with a mixed solvent of petroleum ether:dichloromethane 8:1, obtained a white solid organic room temperature electrophosphorescent material with the structural formula I-25, 0.5 g, and the yield was 76.9%. 1 H NMR (500MHz, CDCl 3 ,δppm):8.75(m,6H),7.59(m,6H),7.43(dd,J=7.6,1.5Hz,1H),7.29(m,1H),7.24(d,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com