Febuxostat impurity
A compound and reaction solvent technology, applied in the field of medicinal chemistry, can solve problems such as no separation and detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] The preparation of embodiment 1 compound 2
[0099]
[0100] Add compound 1 (2.00g) and trifluoroacetic acid (6mL) to the reaction flask, cool down to 20°C, add NBS (1.30g) under stirring at 15-35°C, stir for 5h, then raise the temperature to 35°C and stir for 2h. After the reaction, pour into 50mL of cold water at 10°C to quench, add EA (40mL×2) to extract twice, combine the EA layers, wash the EA layer with 20mL of saturated sodium sulfite solution once, and concentrate and remove the EA layer at 40°C under reduced pressure solvent to obtain compound 1 (2.40 g), with a yield of 100% and a purity of 90%.
[0101] LC-MS: m / z(ESI):424.3(M+H+)+
Embodiment 2
[0102] The preparation of embodiment 2 compound 3
[0103] Add compound 2 (2.40 g) prepared according to the method in Example 1, ethanol (20 mL) to the reaction flask, add a mixed solution of NaOH (1.16 g) and water (8 mL) at room temperature, heat up to 40 ° C and stir for 5 h After the reaction is completed, cool down to 25°C, concentrate under reduced pressure, add 40mL of water, stir and clarify, add aqueous hydrochloric acid (8mL, 2mol / L) to adjust the pH to about 1, continue stirring at 25°C for 2h, filter, and filter the cake with 20mL Washed with water and dried under vacuum at 55°C to obtain compound 3 (2.00 g) with a yield of 87.3% and a purity of 99%.
[0104] LC-MS: m / z (ESI): 395,397 1H NMR (600MHz, DMSO) δ13.46(s, 1H), 8.43(d, J=2.2Hz, 1H), 8.33(d, J=2.2Hz, 1H ), 4.04 (d, J=6.2Hz, 2H), 2.66 (s, 3H), 2.19–2.06 (m, 1H), 0.98 (s, 7H).
Embodiment 3
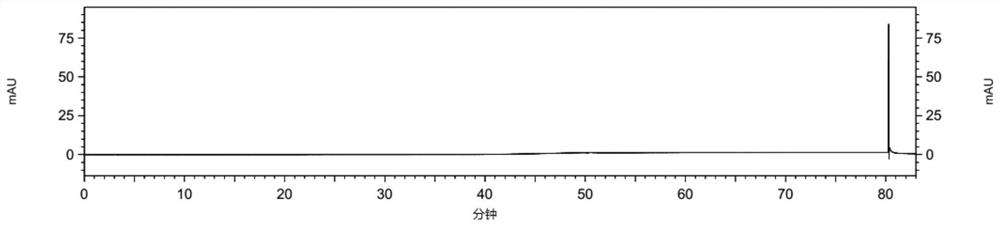
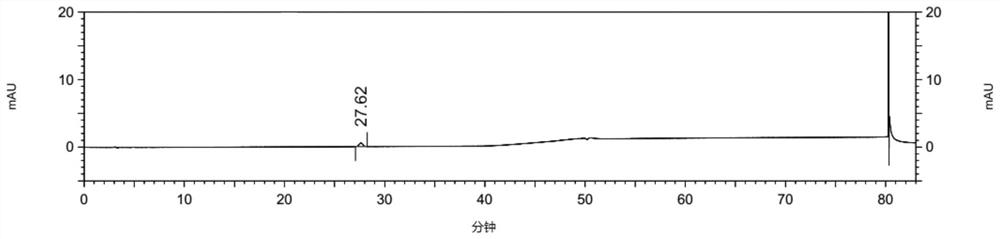
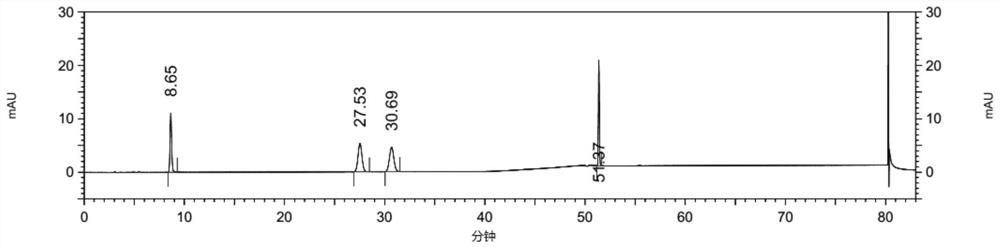
[0105] The detection and analysis of embodiment 3 compound 3
[0106] Instruments and Conditions
[0107]
[0108]
[0109] Experimental procedure
[0110] Solution preparation
[0111] Mobile phase A phase: namely 0.1% trifluoroacetic acid, take 1ml of trifluoroacetic acid and add it to 1000ml ultrapure water, mix well, and filter with 0.2μm water filter membrane to obtain the product;
[0112] Mobile phase B phase: acetonitrile;
[0113] Diluent / blank solution: mixed solution of acetonitrile and water (8:1, V / V);
[0114] Test solution: Take about 25 mg of febuxostat test product, weigh it accurately, put it in a 100ml volumetric flask, add diluent to ultrasonically dissolve and dilute to the mark, shake well, and prepare 2 parts in parallel;
[0115] Control solution: accurately pipette 1ml of the test solution into a 100ml volumetric flask, add diluent to the mark, and shake well; then precisely pipette 5ml of the above solution into a 50ml volumetric flask, dilute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com