Berberine derivative as well as preparation method and application thereof
A derivative, berberine technology, applied in the field of medicinal chemistry, can solve the problems of limited application of berberine, low bioavailability of berberine, etc., and achieves increased ejection fraction and short-axis shortening rate, and the preparation route is feasible and reliable. Reasonable and increase the effect of short axis shortening rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
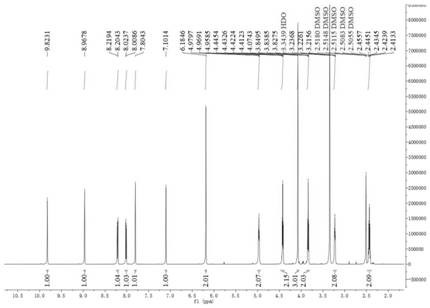
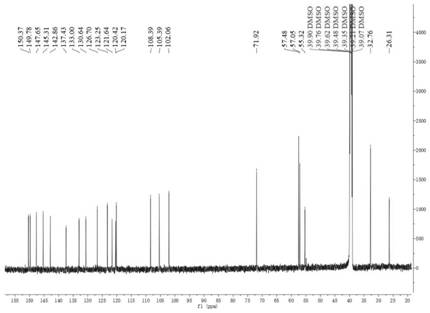
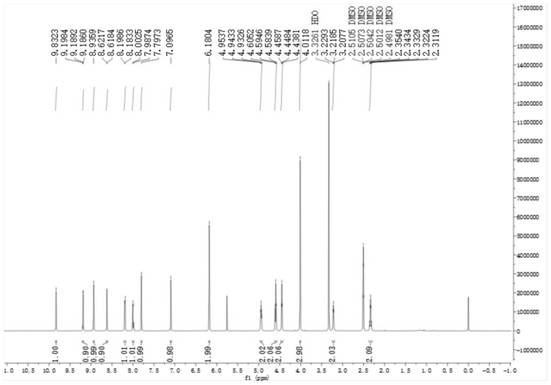
Image
Examples
preparation example Construction
[0100] The fifth aspect of the present invention also provides the preparation method of the berberine derivatives of formula I of the present invention, which comprises the following steps:
[0101]
[0102] Step 1: the compound of formula II reacts with the compound of formula III to generate the compound of formula IV;
[0103] Step 2: reacting the compound of formula IV with the compound of formula V to generate the berberine derivative of formula I;
[0104] Among them, X 1 -X 3 , R 1 -R 3 , L, Het, M - as defined herein;
[0105] T A , T B Independently represents a leaving group, preferably halogen, more preferably chlorine or bromine;
[0106] m 1 - represents an anion, which can be combined with M -Same as M - different. preferred, M 1 - from F - , Cl - 、Br - , I - 、CH 3 COO - .
[0107] When the berberine derivative of formula I is selected from compound 1, the preparation method is:
[0108]
[0109] The reaction of berberine with 1,3-di...
Embodiment 1
[0111] Embodiment 1: the preparation of compound 1 (bromide 9-O-thiazole carboxylate propyl berberine)
[0112] Add 1g of berberine, 0.421mg of potassium carbonate, 0.93g of sodium iodide, 15ml of DMF to the round-bottomed flask in sequence. After reflux at 80°C for 30min, add 850μl of 1,3-dibromopropanol dropwise, continue the reflux reaction for 6h, and spot the plate Monitor the reaction, after the end, add 40ml of ethyl acetate, let stand in ice water for 30min, crystallization precipitates out, filter, after obtaining the crude product, 15ml of DMF is dissolved, then add 30ml of ethyl acetate, secondary recrystallization, suction filtration, drying, to obtain 9-O-bromopropyl berberine bromide 1.31g, yield 90%. Repeat several times to obtain enough intermediates.
[0113] Take 2.019g of 9-O-bromopropyl berberine bromide, 1.356g of thiazole-4-carboxylic acid, 633mg of sodium iodide, 120ml of DMF, and 2.33ml of triethylamine. After reflux reaction at 90°C for 2 hours, th...
Embodiment 2
[0126] Embodiment 2: the cytotoxicity test of compound of the present invention
[0127] 1. Experimental materials
[0128] Experimental cells: primary neonatal mouse cardiomyocytes
[0129] Test substance: bromide 9-O-thiazole carboxylate propyl berberine (compound 1, prepared in Example 1)
[0130] 2. Experimental principle
[0131] The toxicity of the new compound 9-O-thiazolecarboxylate propyl berberine bromide to primary neonatal rat cardiomyocytes was detected by CCK-8 method. CCK-8 reagent contains WST-8: chemical name: 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfone Acid phenyl)-2H-tetrazole monosodium salt, it is reduced to A highly water-soluble yellow formazan product (Formazan). The amount of formazan produced is directly proportional to the number of viable cells. The light absorption value measured at a wavelength of 450nm by an enzyme-linked immunosorbent assay can reflect the number of living cells, and the larger the value, the stronger th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com