Synthesis method and application of nitrogen heterocyclic compound with anti-tumor effect
A technology for a nitrogen heterocyclic compound and a synthesis method, which is applied in the field of biomedicine, can solve the problems of limited operation steps, difficulty in obtaining o-phenylenediamine substrates, and limited product diversity, and achieves single selectivity and high reaction yield. , the effect of excellent atom economy and step economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
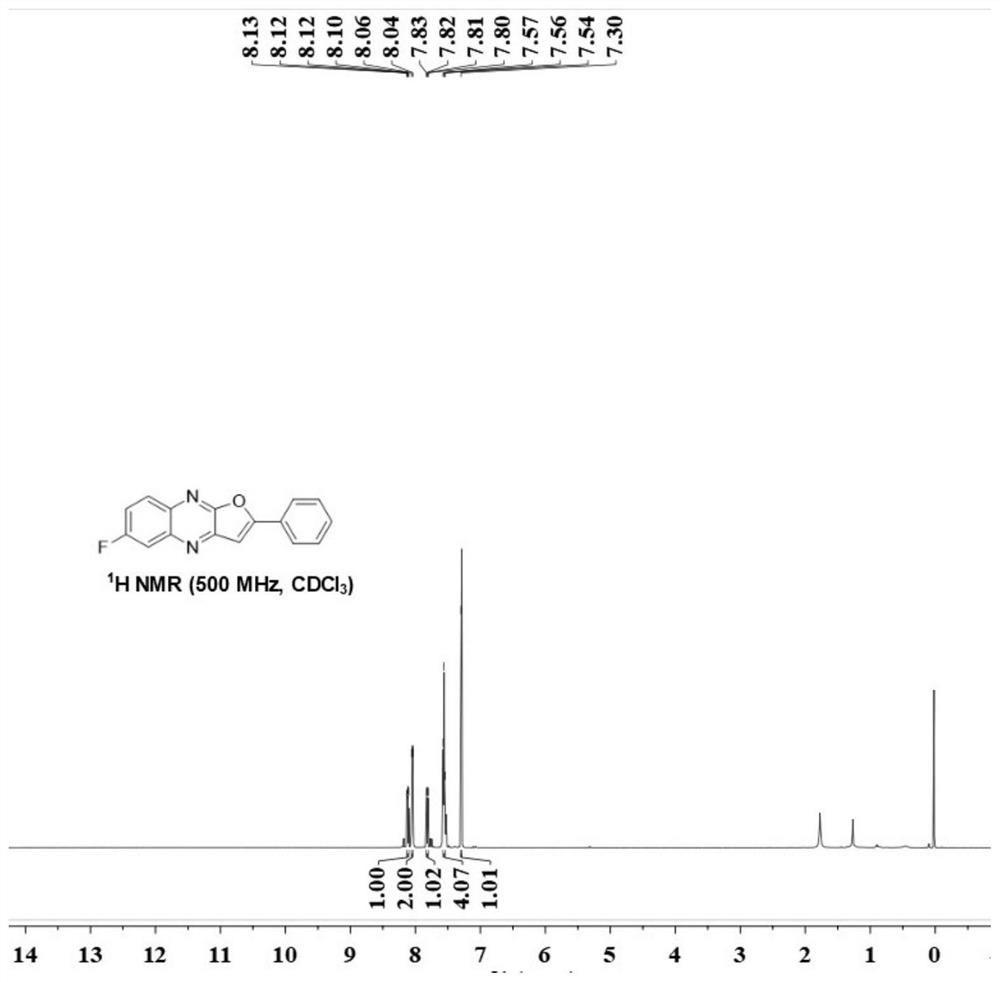
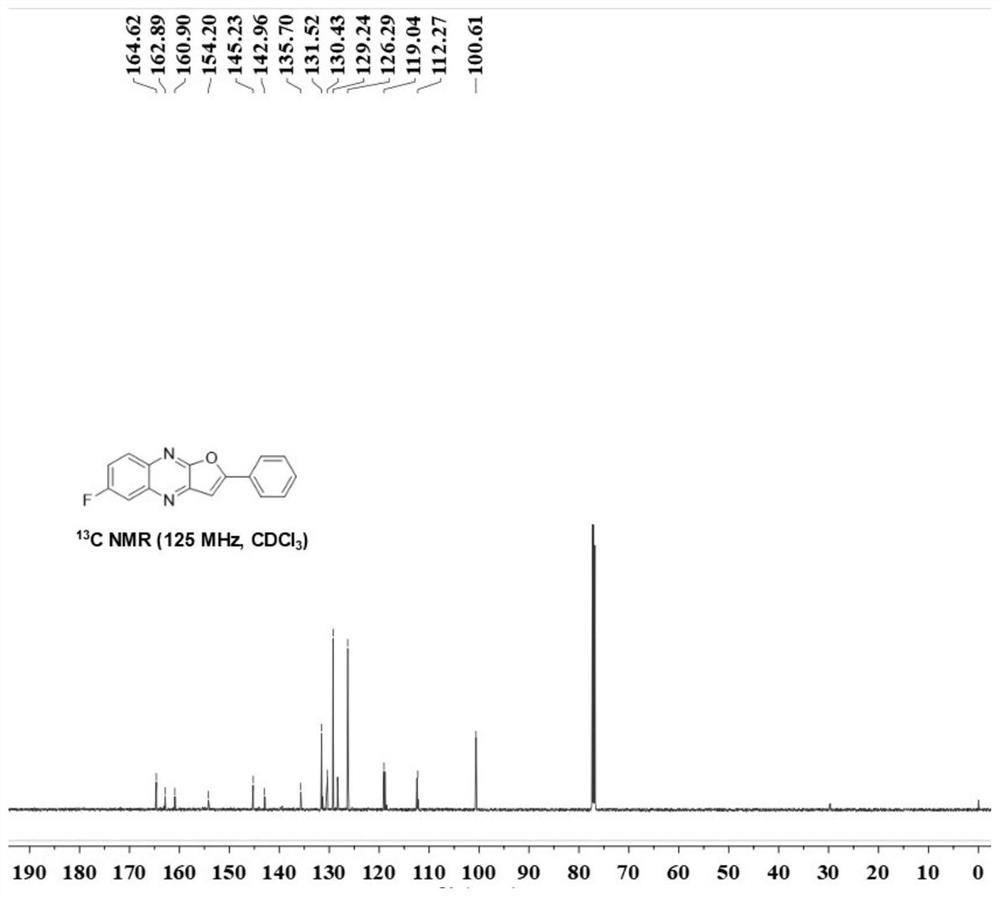
[0025] (1) Weigh acetophenone (0.4mmol), 6-fluoroquinoxaline-2(1H)-one (0.2 mmol), copper trifluoromethanesulfonate (0.03mmol), K 2 S 2 o 8 (0.6mmol) and boric acid (0.4 mmol), transferred to a three-necked flask of 10 ml, added 2 ml of 1,2-dichloroethane, stirred magnetically at 80 degrees Celsius for 12 hours, cooled to room temperature after the reaction, filtered and collected The organic phase was rotary evaporated under reduced pressure to obtain the crude product 6-fluoro-2-phenylfuro[2,3-b]quinoxaline (A), and finally the pure target product was obtained by column chromatography. The eluent is petroleum ether: ethyl acetate = 3: 1, and the separation yield: 85%. Yellow solid; melting point 217.2-218.2°C; IR: ν=3432,1630, 819,757,730,681,658,614,429cm -1 ; 1 H NMR (500MHz, Chloroform-d) δ8.12(dd, J=9.2,5.7Hz,1H),8.05(d,J=9.6Hz,2H), 7.82(dd,J=9.5,2.8Hz,1H) ,7.56(t,J=6.8Hz,4H),7.30(s,1H); 13 C NMR (125MHz, Chloroform-d) δ164.6, 161.9 (q, J = 248.8Hz), 154.2, 145.2, ...
Embodiment 2
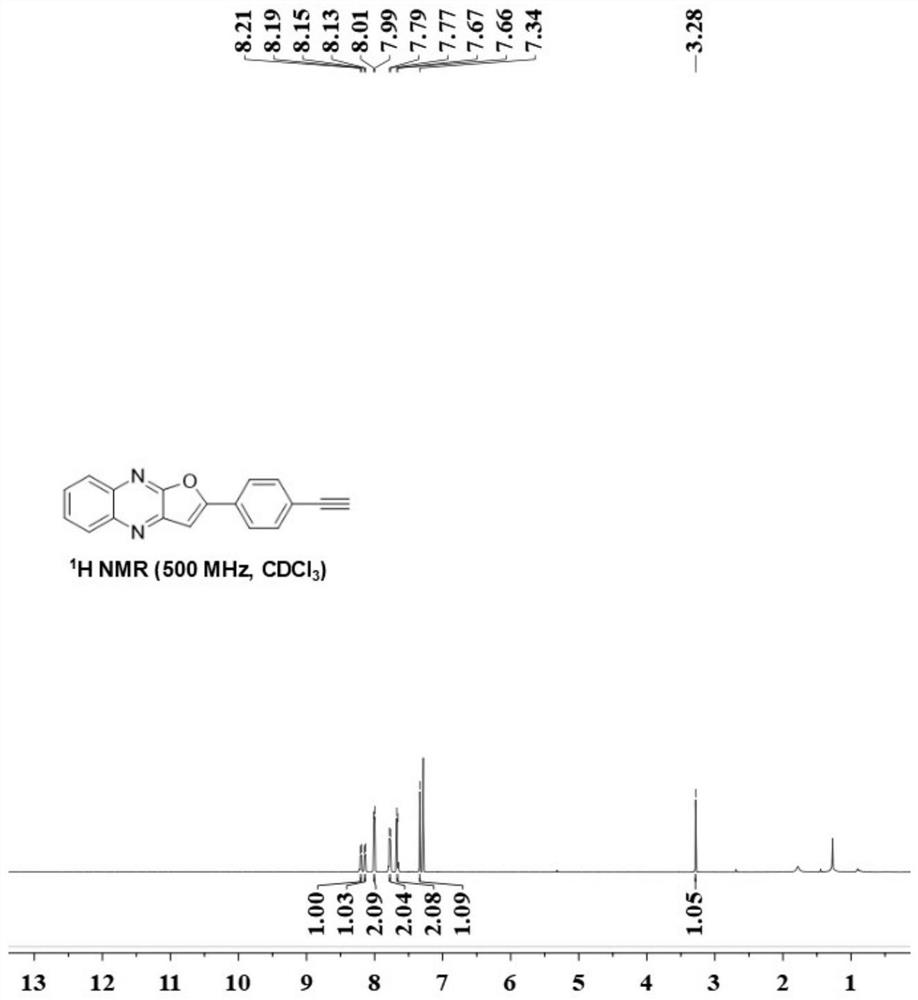
[0027] (2) Weigh 4-ethynyl acetophenone (0.4mmol), quinoxalin-2(1H)-one (0.2 mmol), copper trifluoromethanesulfonate (0.03mmol), K 2 S 2 o 8 (0.6mmol) and boric acid (0.4 mmol), transferred to a three-necked flask of 10 ml, added 2 ml of 1,2-dichloroethane, stirred magnetically at 80 degrees Celsius for 12 hours, cooled to room temperature after the reaction, filtered and collected The organic phase was rotary evaporated under reduced pressure to obtain the crude product 2-(4-ethynylphenyl)furo[2,3-b]quinoxaline (B), and finally the pure target product was obtained by column chromatography. The eluent is petroleum ether:ethyl acetate=3:1, and the separation yield: 87%. Brown solid; melting point 191.6-192.6°C; IR: ν = 3454, 1633, 750, 534cm -1 ; 1 H NMR (500MHz, Chloroform-d) δ8.20(d, J=9.8Hz, 1H), 8.13(s, 1H), 8.00(d, J=8.5Hz, 2H), 7.78(d, J=9.8Hz , 2H), 7.67(d, J=8.5Hz, 2H), 7.34(s, 1H), 3.28(s, 1H); 13 C NMR (125 MHz, Chloroform-d) δ162.9, 154.4, 144.3, 142.3, 139.0, ...
Embodiment 3
[0029] (3) Weigh cyclohexyl ethyl ketone (0.4mmol), quinoxalin-2(1H)-one (0.2mmol), copper trifluoromethanesulfonate (0.03mmol), K 2 S 2 o 8 (0.6mmol) and boric acid (0.4mmol), transferred to a three-necked flask of 10 ml, added 2 ml of 1,2-dichloroethane, magnetically stirred at 80 degrees Celsius for 12 hours, cooled to room temperature after the reaction, filtered and collected The organic phase was rotary evaporated under reduced pressure to obtain the crude product 2-cyclopropylfuro[2,3-b]quinoxaline (C), and finally the pure target product was obtained by column chromatography. The eluent is petroleum ether:ethyl acetate=3:1, and the separation yield: 75%. Dark black solid; melting point 90.1-91.1°C; IR: ν=3445,1632,1569,1385, 1311,941,806,760,599,454cm -1 ; 1 H NMR (500MHz, Chloroform-d) δ8.15(d, J=9.8Hz, 1H), 8.07(d, J=7.7Hz, 1H), 7.74–7.70(m, 2H), 6.73(s, 1H) ,2.24–2.20(m,1H),1.33(dd,J=4.8,2.5Hz,2H),1.25–1.22(m,2H); 13 C NMR (125MHz, Chloroform-d) δ170.9, 153.9,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com