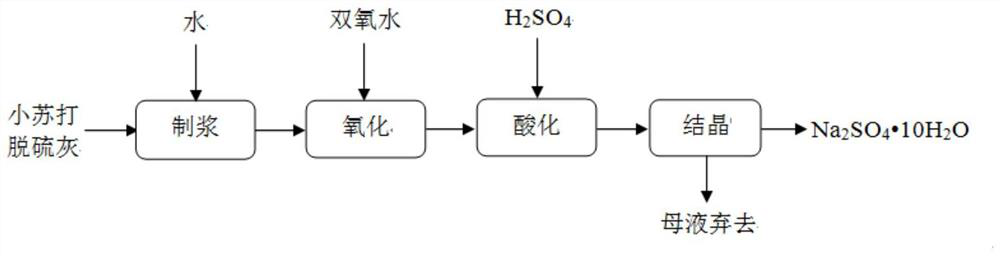
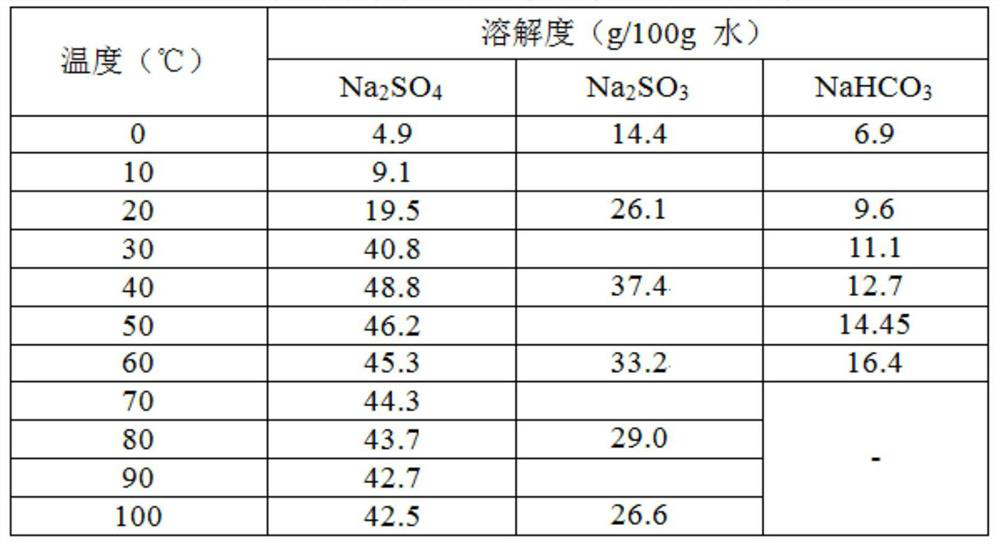
Method for preparing sodium sulfate from baking soda desulfurized fly ash by wet process
A desulfurization ash and baking soda technology, applied in the direction of sulfate/bisulfate preparation, etc., can solve the problems of low reaction efficiency, high dust content, affecting product purity, etc., and achieve simple process flow, cheap and easy-to-obtain equipment, and economic benefits obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) The baking soda desulfurization ash to be disposed of is sampled and analyzed, and the results show that it consists of: Na 2 SO 4 32%, Na 2 SO 3 11%, NaHCO 3 54%, other components are a small amount of crystal water and / or unavoidable impurities.
[0035] 2) Put 200g of baking soda desulfurization ash into a stirring tank, add 200g of pure water, heat the mixture to 34°C to prepare a suspension, and stir for 12min.
[0036] 3) Add 24g of 30wt% hydrogen peroxide solution (equivalent to H2) in the stirring tank 2 o 2 about 0.21mol), and kept stirring at 34°C for 20min.
[0037] 4) add 25wt%% dilute sulfuric acid solution 327g (equivalent to H 2 SO 4 about 0.83mol), and kept stirring at 34°C for 7min.
[0038] 5) The mixture is transferred to the crystallizer, and the calculation shows that Na in the reacted mixture 2 SO 4 The mass is about 180g. In order to ensure product purity and yield, the mixture was heated to evaporate part of the water until the ...
Embodiment 2
[0041] 1) The baking soda desulfurization ash to be disposed of is sampled and analyzed, and the results show that it consists of: Na 2 SO 4 58%, Na 2 SO 3 18%, NaHCO 3 22%, other components are a small amount of crystal water and / or unavoidable impurities.
[0042] 2) Take 250g of baking soda desulfurization ash and put it in a stirring tank, then add 300g of pure water, heat the mixture to 46°C to prepare a suspension, and stir for 12min.
[0043] 3) Add 58g of 30wt% hydrogen peroxide solution (equivalent to H2) in the stirring tank 2 o 2 about 0.51mol), and kept stirring at 46°C for 40min.
[0044] 4) add 50wt% dilute sulfuric acid solution 83g (equivalent to H 2 SO 4 About 0.42mol), keep stirring at 46°C for 12min.
[0045] 5) The mixture is transferred to the crystallizer, and the calculation shows that Na in the reacted mixture 2 SO 4 The mass is about 242g. In order to ensure product purity and yield, the mixture was heated to evaporate part of the water ...
Embodiment 3
[0048] 1) The baking soda desulfurization ash to be disposed of is sampled and analyzed, and the results show that it consists of: Na 2 SO 4 45%, Na 2 SO 3 14%, NaHCO 3 38%, other components are a small amount of crystal water and / or unavoidable impurities.
[0049] 2) Take 300g of baking soda desulfurization ash and put it in a stirring tank, then add 370g of pure water, heat the mixture to 40°C to prepare a suspension, and stir for 12min.
[0050] 3) Add 49g (equivalent to H) of 30wt% hydrogen peroxide solution in the stirring tank 2 o 2 about 0.43mol), and kept stirring at 40°C for 32min.
[0051] 4) add 40wt% dilute sulfuric acid solution 198g (equivalent to H 2 SO 4 about 0.81mol), and kept stirring at 40°C for 10min.
[0052] 5) The mixture is transferred to the crystallizer, and the calculation shows that Na in the reacted mixture 2 SO 4 The mass is about 279g. In order to ensure product purity and yield, the mixture was heated to evaporate part of the wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com