Recombinant human fibroblast growth factor-9 protein drug freeze-dried preparation and application thereof
A technology of human fibroblasts and fibroblasts, applied in freeze-drying delivery, drug combination, drug delivery and other directions, can solve the problems of difficult to use biological agents, easy to break, unstable peptide bonds, etc., to protect the antioxidant capacity , reducing inflammation and protecting protein stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 Preparation method of recombinant human fibroblast growth factor-9 protein drug freeze-dried preparation Solution preparation and filling method of freeze-dried preparation solution with different proportions:
[0023] 1) Preparation of protein solution: the purified FGF9 protein was dialyzed in 25mM citrate buffer (pH 6.0) and concentrated by ultrafiltration to a protein concentration of 6.5mg / ml, sterilized with a sterile 0.22μm microporous membrane Use after filtering. The above operations are all carried out at 2-8 °C;
[0024] 2) Preparation of auxiliary material solution: accurately weigh each component according to the formulas designed in Table 1 below, and fully dissolve it with 25mM citrate buffer (pH6.0), adjust the pH to 6.0, and use sterile 0.22μm After sterilization and filtration by microporous membrane, place it at 2-8℃ to pre-cool for use;
[0025] 3) Mixing the protein solution and the auxiliary material solution 1:1 (v / v) to make the pr...
Embodiment 2
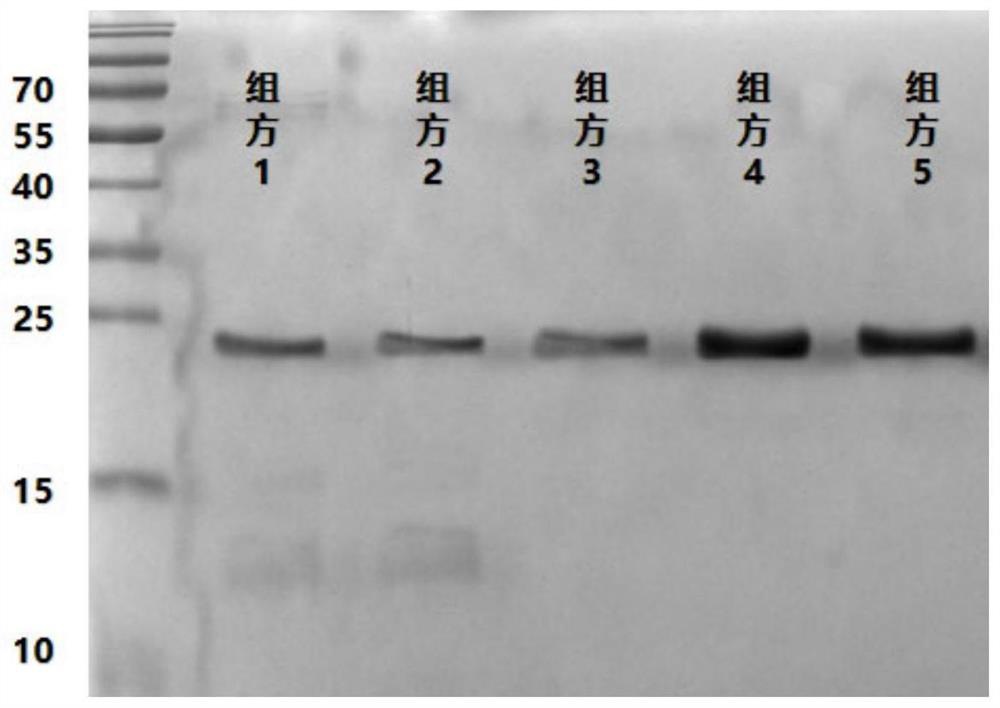
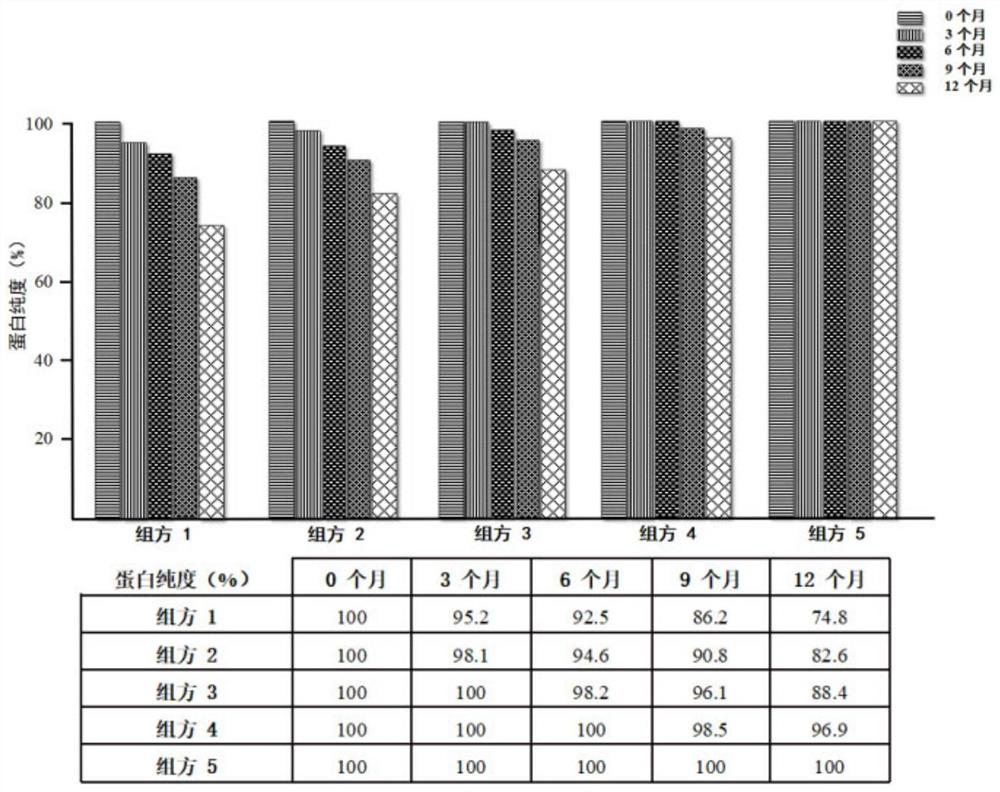
[0034] Example 2: Detection of the stability of recombinant human fibroblast growth factor-9 protein drug freeze-dried preparations of each prescription by non-reducing SDS-PAGE gel electrophoresis
[0035] The protein purity of different formulations at 0, 3, 6, 9, and 12 months was detected by non-reducing SDS-PAGE gel electrophoresis under the storage conditions of 5±3°C and RH 65%. The specific operations are as follows:
[0036] 1) Electrophoresis protein sample preparation: The preserved FGF9 protein lyophilized preparation was dissolved with water for injection at each time point and diluted to 1 mg / ml. Precisely pipette 60 μl, add 20 μl 4× non-reducing sample treatment solution, mix well, boil at 100°C for 10 min, and centrifuge at 13000 rpm for 10 min.
[0037] 2) Electrophoresis gel preparation: 12% separating gel was prepared according to the SDS-polyacrylamide gel electrophoresis method in the general rule of the Pharmacopoeia, capped with water, and polymerized at ...
Embodiment 3
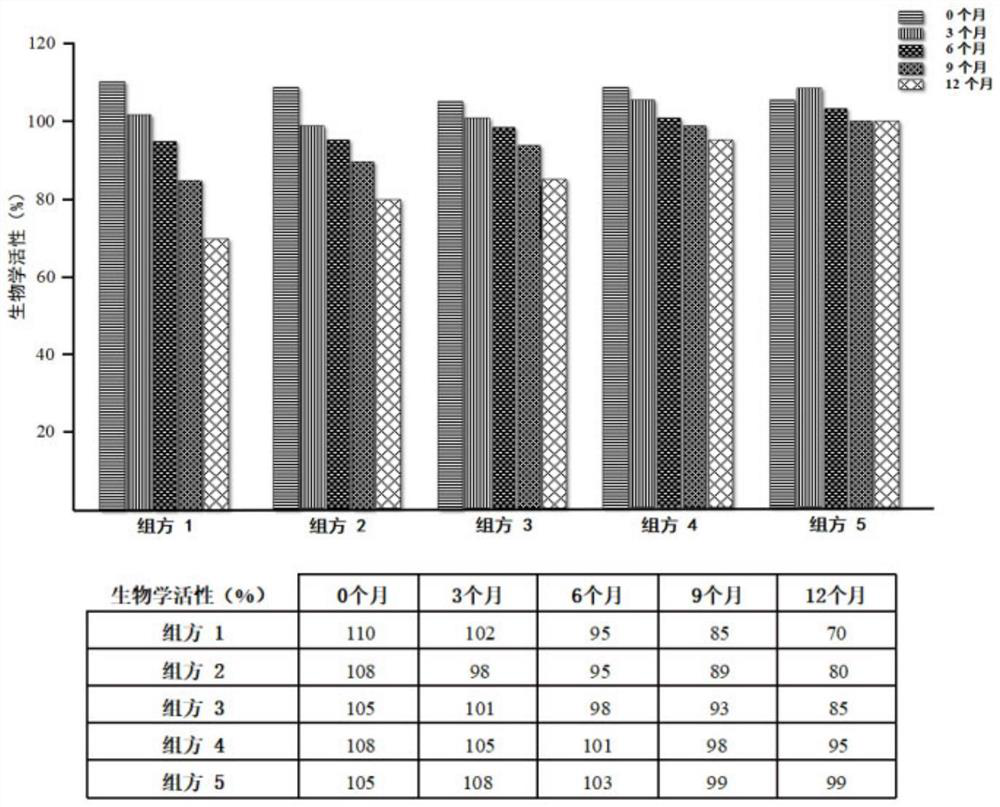
[0044] Example 3: Detection of the stability of recombinant human fibroblast growth factor-9 protein drug freeze-dried preparations of each prescription by cell proliferation method / MTT colorimetric method
[0045] Using NIH / 3T3 cells as the detection cell line, the biological activity of different formulations at 0, 3, 6, 9, and 12 months was determined by cell proliferation method / MTT colorimetric method under the storage conditions of 5±3℃ and RH 65%. academic activity. The specific operations are as follows:
[0046] 1) Preparation of standard solution: Take one fibroblast growth factor standard product purchased by the China Inspection and Quarantine, dissolve it with 1ml of maintenance culture medium and dispense it to 40IU / ml. In a 96-well cell culture plate, make 4-fold serial dilutions with 2 wells per dilution. The above operations were carried out under sterile conditions.
[0047] 2) Preparation of test solution: Dilute FGF9 protein with maintenance medium to ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com