Bimetal MOF (Metal Organic Framework) derived catalyst as well as preparation method and application thereof
A catalyst, bimetallic technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problems of difficult formation of active sites, few exposed active sites, poor catalytic stability, etc., and achieve high yield, excellent performance, secondary less reactive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] In the second aspect, the embodiment of the present application provides a method for preparing a bimetallic MOF-derived catalyst, comprising the following steps:
[0053] S1: The first intermediate is obtained by modifying the bimetallic MOF and the non-ionic polymer compound in an organic solvent;
[0054] S2: Mixing and reacting the first intermediate with a soluble zinc salt and a nitrogen-containing ligand in an organic solvent to obtain a second intermediate;
[0055] S3: carbonizing the second intermediate to obtain a third intermediate;
[0056] S4: Mixing the third intermediate with a fluorinating agent for fluorination treatment to obtain a catalyst.
[0057] In the preparation method of the bimetallic MOF-derived catalyst provided in the second aspect of the embodiment of the present application, the nitrogen-doped carbon nanotubes attached to the surface of the synthesized catalyst are loose and not dense, which helps to expose the metal catalytic active ce...
Embodiment 1
[0082] Preparation of FeNi@NCNT-F electrooxidation oxygen generation catalyst:
[0083] In the first step, add 905mg of ferric chloride, 480mg of nickel nitrate and 831mg of terephthalic acid into 50mL of N,N-dimethylformamide solution in turn, then add 20mL of 0.2M NaOH solution, stir at room temperature until mixed uniform. The above solution was transferred to a Teflon substrate with a volume of 100mL, and the temperature of the oven was set at 100°C, and the holding time was 15h. After the reaction was completed and cooled to room temperature, it was washed with deionized water and ethanol several times, centrifuged, and vacuum-dried to obtain a yellow powder, which was tested by a powder XRD diffractometer to obtain FeNi MIL MOF, which was used in the next experiment.
[0084] In the second step, 800 mg of the synthesized FeNi MIL MOF was taken at room temperature, and 4 g of polyvinylpyrrolidone was dissolved in 80 mL of methanol solution and stirred for 15 h.
[0085]...
Embodiment 2
[0092] Preparation of FeNi@NCNT-F-1-6 electrooxidation catalyst:
[0093] The preparation process of this example is the same as that of Example 1, the difference being that the molar ratio of zinc nitrate to 2-methylimidazole is 1:6; specifically in the second step, the preparation of solution C is 48 mmol of 2-methylimidazole solution Obtain solution C in 100mL methanol solution, mix solutions A, B, and C for 5 minutes and then let it stand for 24 hours. The molar ratio of zinc nitrate to 2-methylimidazole is 1:6.
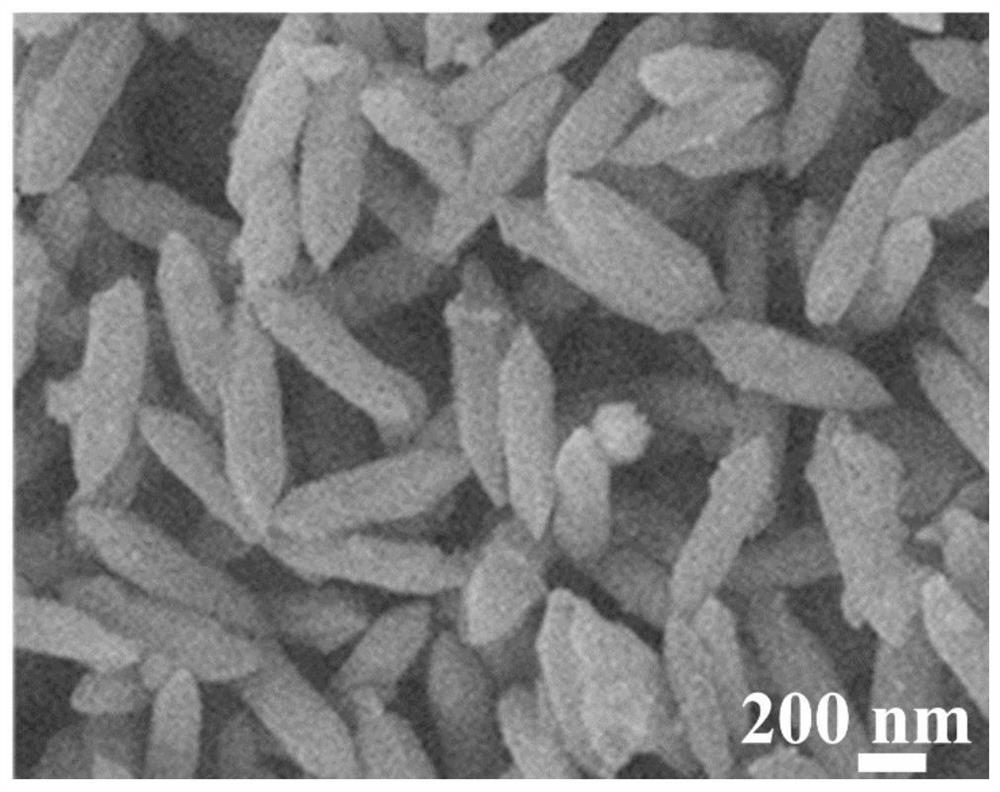
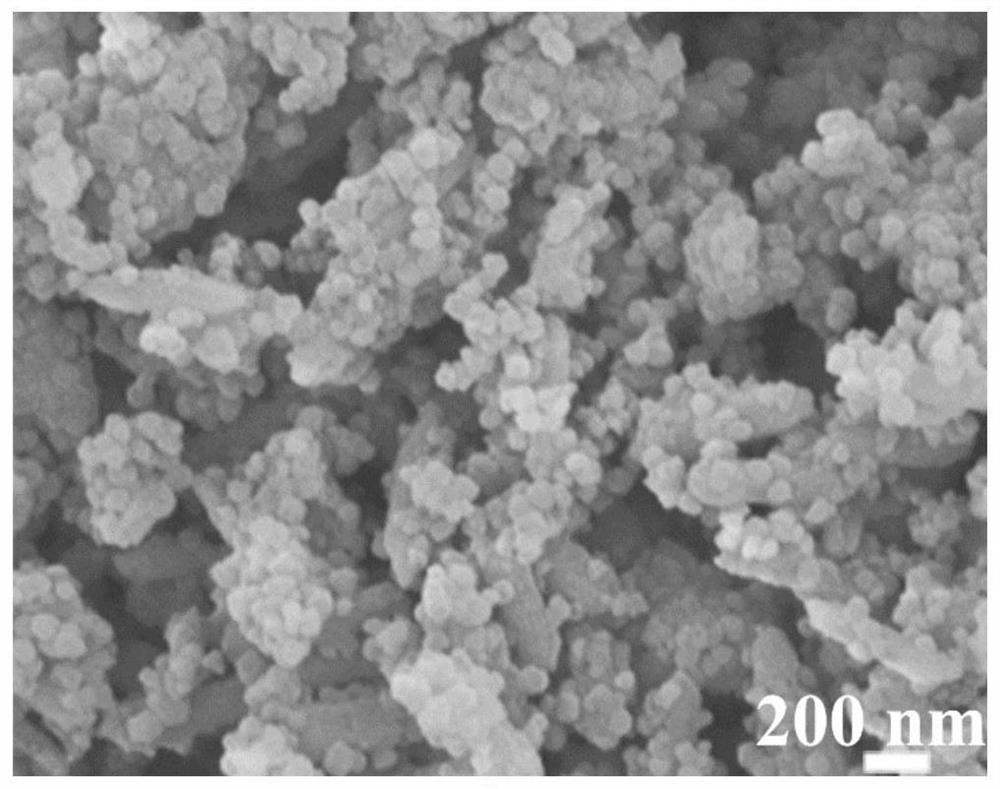
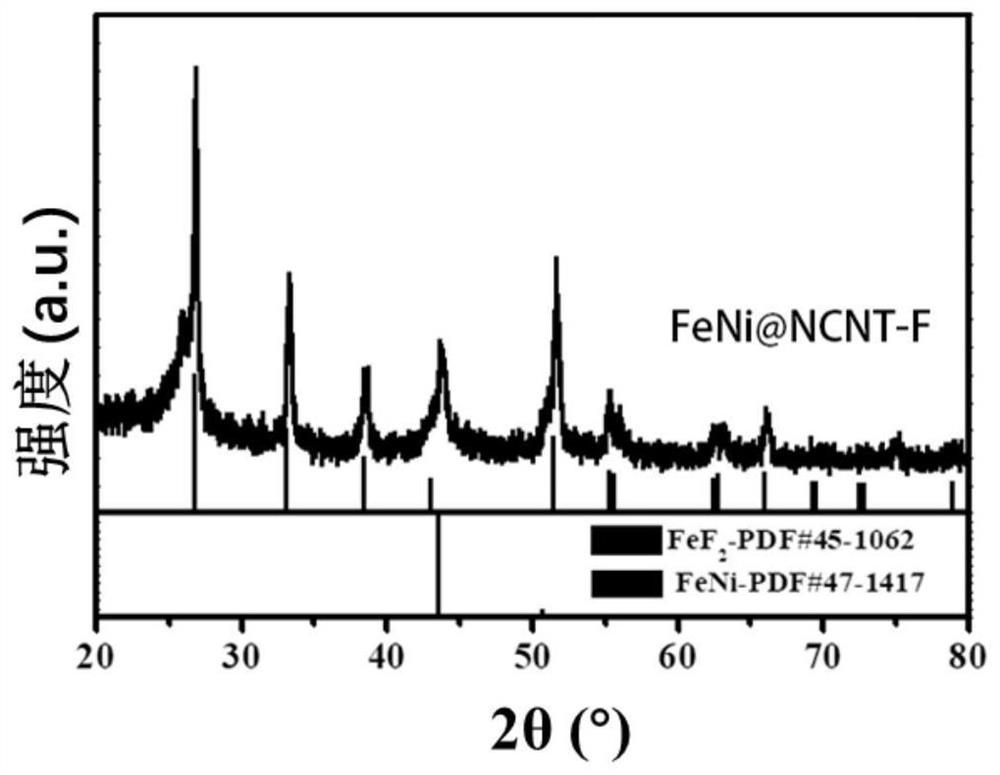
[0094] Figure 6 It is the SEM image of FeNi MIL@ZIF 8-1-6MOF prepared in Example 2. It can be seen that the particles of ZIF 8 are relatively large and cover the surface of FeNi MIL MOF. Figure 7 and Figure 8 It is the SEM image and XRD image of FeNi@NCNT-F-1-6 after carbonization and fluorination, the ZIF 8 covered on the surface is converted into CNT, but agglomeration can still be observed. FeNi MIL@ZIF 8-1-6MOF in N 2 After carbonization pyrolysis and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com