Composition and medicine for treating cardiovascular and cerebrovascular diseases as well as preparation method and application of composition and medicine
A technology for cardiovascular and cerebrovascular diseases and compositions, which is applied in the field of compositions for the treatment of cardiovascular and cerebrovascular diseases. quick results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
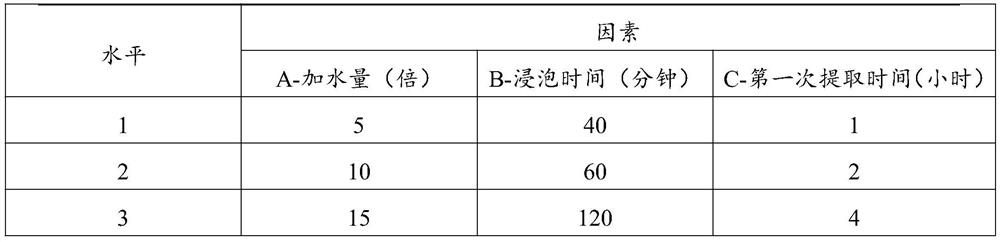
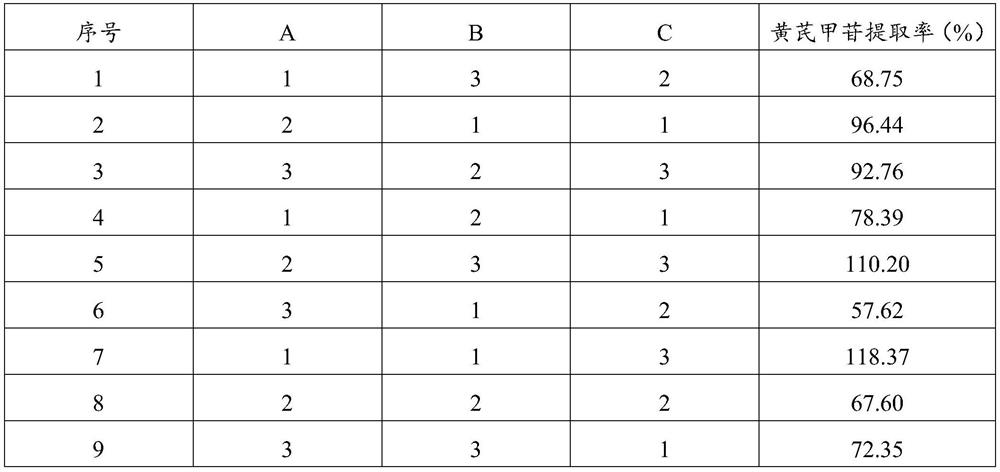
[0033] Embodiment 1, investigate the impact of different water extraction process conditions on active ingredients
[0034] The composition of the prescription is as follows:
[0035] Saffron whole flower 70g, rhodiola rosea 200g, sumac 150g, angelica 200g, dannanxing 50g, chuanxiong 200g, astragalus 300g, earthworm 150g, achyranthes bidentata 150g.
[0036] The preparation method is as follows:
[0037] 1) Decoct the prescribed amount of Astragalus membranaceus, Earthworm, Sappan, and Achyranthes bidentata with water for three times, combine the three extracts, filter, concentrate to a thick paste with a relative density of 1.30-1.35 (50°C), and dry at 90°C , pulverized to obtain medicinal powder 1;
[0038] 2) Rhizoma Chuanxiong, Angelica, Rhodiola (consumption is 6 times of the volume of Rhizoma Chuanxiong, Angelica, Rhodiola) of the prescribed amount were soaked for 60 minutes, then extracted three times with a volume fraction of 70% ethanol aqueous solution (adding 6 fo...
Embodiment 2
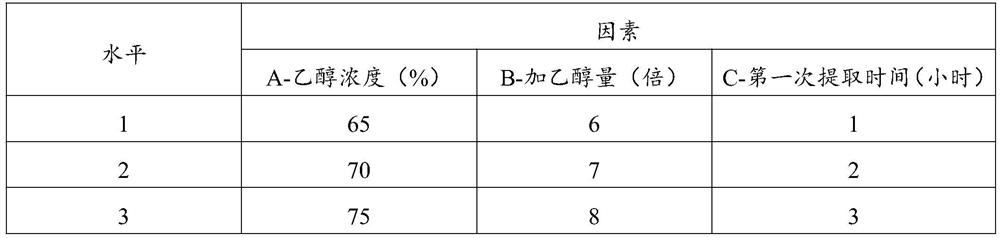
[0048] Embodiment 2, investigate the impact of different alcohol extraction process conditions on active ingredients
[0049] The composition of the prescription is the same as in Example 1.
[0050] The preparation method is as follows:
[0051] 1) Decoct the prescription amount of Astragalus membranaceus, Earthworm, Sappan, and Achyranthes bidentata with water three times (add 10 times the volume of water, soak for 120 minutes, and add water for three decoctions in order of the above-mentioned Astragalus, Earthworm, and Suxie). 10 times, 7 times, and 4 times the volume of Achyranthes chinensis and Achyranthes chinensis; the time of decocting three times was 4 hours, 2 hours, and 1 hour in turn.), the three extractions were combined, filtered, and concentrated to a relative density of 1.30 to 1.35 ( 50°C) thick paste, dried at 90°C and pulverized to obtain medicinal powder 1;
[0052] 2) Extract the prescription amount of Rhizoma Chuanxiong, Angelica, and Rhodiola three tim...
Embodiment 3
[0062] Embodiment 3, the drug effect comparison after the different parts of saffron are used as medicine
[0063] According to the different drug parts of saffron, the following two Jingzhu Shuanghong Huoxue capsule preparations were prepared respectively, and their protective effects on myocardial ischemia-reperfusion injury in rats were observed.
[0064] (1) Preparation A: 70g of saffron whole flower, 200g of rhodiola rosea, 150g of sumac, 200g of angelica, 50g of Dannanxing, 200g of Chuanxiong, 300g of astragalus, 150g of earthworm, 150g of Achyranthes bidentata, prepared according to the method of the above-mentioned embodiment 1 .
[0065] (2) B preparation: Saffron stigma 5g, Rhodiola rosea 200g, Sappan 150g, Angelica 200g, Dannanxing 50g, Rhizoma Chuanxiong 200g, Astragalus 300g, Dilong 150g, Achyranthes bidentata 150g, prepared according to the method of above-mentioned embodiment 1.
[0066] The results are shown in Table 5. ATP / ADP: A preparation group was signif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com