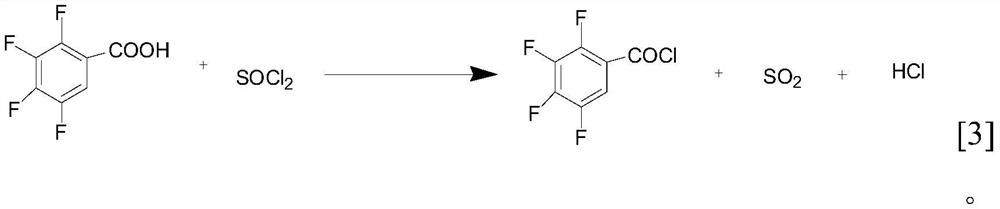
Efficient and green synthesis method of 2, 3, 4, 5-tetrafluorobenzoyl chloride
A technology for tetrafluorobenzoyl chloride and green synthesis, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve the problems of low production yield, achieve increased yield, and increase product yield , The effect of less damage to the reaction material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
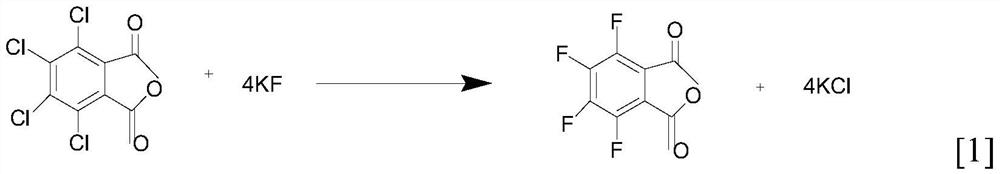
[0022] Put 200ml of toluene into the reaction kettle, put in 45g of potassium fluoride, 1g of catalyst tetramethylammonium chloride, and 50g of tetrachlorophthalic anhydride, raise the temperature to 100°C, then seal it and raise the temperature to 120-150°C, fill it with nitrogen to 0.4MPa, and carry out Insulate and react for 5 hours, take a sample for liquid chromatography detection, the spectrum shows that the peak purity of tetrachlorophthalic anhydride of the raw material is ≤1%, and then cool down to 50°C for pressure filtration to remove potassium chloride, potassium chloride is dried as a by-product, and the toluene mother liquor is removed Next reaction.
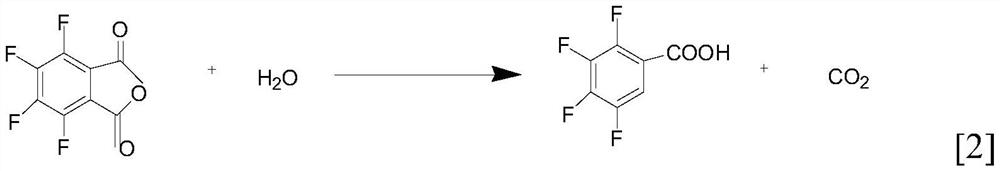
[0023] Transfer the toluene filtrate from the previous step to the decarboxylation reactor, control the temperature at about 45°C, add 18g of water (use 1 batch of water for one layering, add 3.5g of water), 12.5g of sodium carbonate (use 1 batch of sodium carbonate, add new Sodium carbonate 1g), warming up to 75-8...
Embodiment 2
[0026] Put 150ml of toluene into the reaction kettle, put in 50g of potassium fluoride, 1g of catalyst tetramethylammonium chloride, and 50g of tetrachlorophthalic anhydride, raise the temperature to 100°C, then seal it and raise the temperature to 120-150°C, fill it with nitrogen to 0.4MPa, and carry out Insulate and react for 5 hours, take a sample for liquid chromatography detection, the spectrum shows that the peak purity of tetrachlorophthalic anhydride of the raw material is ≤1%, and then cool down to 50°C for pressure filtration to remove potassium chloride, potassium chloride is dried as a by-product, and the toluene mother liquor is removed Next reaction.
[0027] Transfer the toluene filtrate from the previous step to the decarboxylation reaction kettle, control the temperature at about 45°C, add 17g of water (use 1 batch of water for one layering, add 3.0g of water), 12.5g of sodium carbonate (use 1 batch of sodium carbonate, add new Sodium carbonate 1g), warming up...
Embodiment 3
[0030] Put 250ml of toluene into the reaction kettle, put 40g of potassium fluoride, 1g of catalyst tetramethylammonium chloride, and 50g of tetrachlorophthalic anhydride, raise the temperature to 100°C, then seal it and raise the temperature to 120-150°C, fill it with nitrogen to 0.4MPa, and carry out Insulate and react for 5 hours, take a sample for liquid chromatography detection, the spectrum shows that the peak purity of tetrachlorophthalic anhydride of the raw material is ≤1%, and then cool down to 50°C for pressure filtration to remove potassium chloride, potassium chloride is dried as a by-product, and the toluene mother liquor is removed Next reaction.
[0031] Transfer the toluene filtrate from the previous step to the decarboxylation reaction kettle, control the temperature at about 45°C, add 20g of water (use 1 batch of water for one layering, add 4.0g of water), 12.5g of sodium carbonate (use 1 batch of sodium carbonate, add new Sodium carbonate 1g), warming up to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com