Vanillic acid farnesyl ester, preparation method thereof and application of vanillic acid farnesyl ester in tobacco industry
A technology of farnesyl and vanillic acid, applied in farnesyl vanillic acid and its preparation, and the application field in the tobacco industry, which can solve problems such as injury, and achieve good fluidity, sweet smoke, rich and harmonious aroma Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
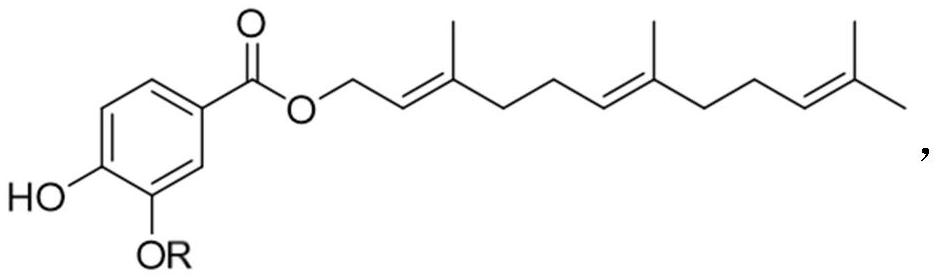
[0032] Example 1: (2E,6E)-3,7,11-trimethyldodecyl-2,6,10-trien-1-yl 4-hydroxy-3-methoxybenzoate ( Preparation of VF)
[0033]
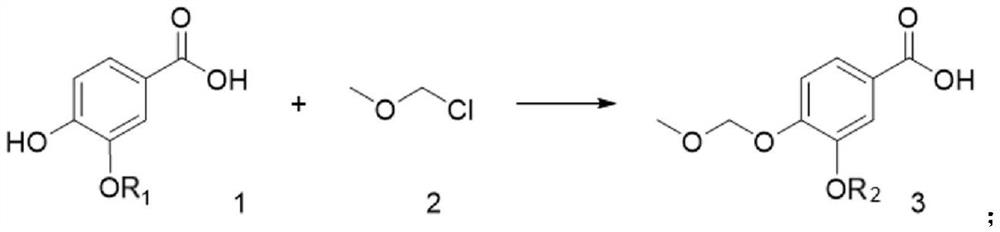
[0034] The compound of formula 1 (10mmol, 1eq) was dissolved in 10mL of tetrahydrofuran, and the compound of formula 2 (12mmol, 1.2eq), 500μL of triethylamine and (1mmol, 0.1eq) potassium iodide were added, and reacted at room temperature for 12h. After the reaction was complete, the solvent was concentrated by evaporation, extracted with saturated brine and DCM, and the organic layer was dried over anhydrous sodium sulfate and concentrated by evaporation to obtain the compound of formula 3 with a yield of 89%. MS(ESI)m / z:213[M+H] + .
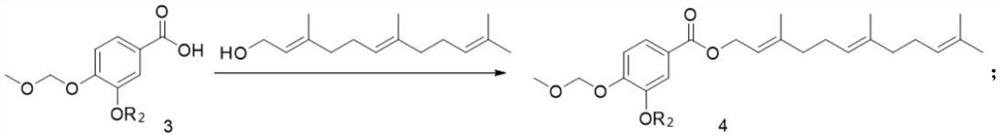
[0035] The compound of formula 3 (10mmol, 1eq) was dissolved in 10mL DCM, and farnesol (12mmol, 1.2eq), EDCI (12mmol, 1.2eq) and DMAP (5mmol, 0.5eq) were added, and reacted at room temperature for 12h. After the reaction was complete, the solvent was concentrated, extracted with saturated saline and DCM, and the ...
Embodiment 2
[0036] Embodiment 2: the preparation of farnesyl vanillic acid fragrance preparation
[0037] The purified product VF (purity > 99%) prepared in Example 1 is added to a mixing tank, and an emulsifier (by glyceryl monostearate, glyceryl caprylate, Tween 80 in a weight ratio of 1:1:0.5) is added to the mixing tank Composition), stirring and mixing at a certain temperature for 0.5h, cooling the feed liquid, transporting it to a high-pressure homogenizer, and then uniformly mixing with 80% ethanol solution to obtain the fragrance preparation of farnesyl vanillic acid described in the patent of the present invention.
[0038] The added amount of the farnesyl vanillate is 45%-49.999% of the quality of the fragrance preparation. The added amount of the emulsifier is 0.001-5% of the mass of the fragrance preparation; the mixing conditions are temperature 60-80°C, stirring speed 200-350rpm; high-pressure homogenization conditions are feed temperature 45°C, homogenization pressure 10-20...
Embodiment 3
[0039] Embodiment 3: Appearance, solubility, smell, and sensory evaluation of the farnesyl vanilloid fragrance preparation of the present invention
[0040] Taking the farnesyl vanillic acid fragrance preparation of Example 2 as an example, the appearance, smell, and sensory evaluation were carried out, and the results are as follows:
[0041] Appearance status: clarified, translucent, no delamination after standing.
[0042] Smell sense: light fruity woody fragrance, sweet and sweet fragrance, less irritating fragrance, different from natural flavors such as vanillic acid, geraniol, farnesol, etc., which have the disadvantages of strong volatility and relatively irritating fragrance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com