Novel method for synthesizing cholesterol and 25-hydroxycholesterol by taking 22-sterol as raw material
A technology for hydroxycholesterol and cholesterol, applied in the field of steroid synthesis, can solve the problems of inconsistent atom economy, high reaction risk, etc., and achieve the effects of environmental protection, simple reaction process and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
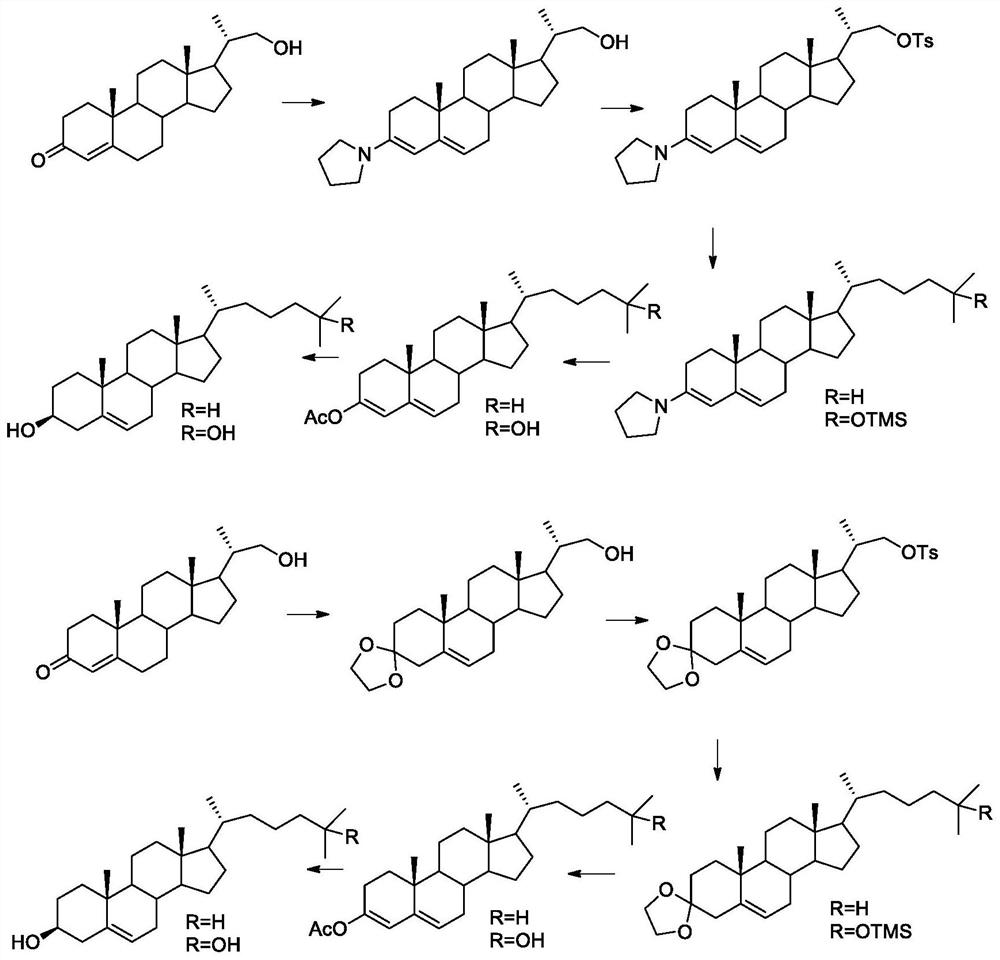
[0040] The synthetic method of cholesterol and 25-hydroxyl cholesterol, as attached figure 1 shown, including the following steps:
[0041] 1. 3-position carbonyl tetrahydropyrrole protection
[0042] Put 1000g of 22-hydroxy-20-methylpregn-4-en-3-one and 5000ml of anhydrous methanol into a 10L three-necked flask, add 200g of tetrahydropyrrole dropwise under heating and reflux, the reaction solution becomes turbid again, and the dropwise addition is completed After the reaction was cooled to room temperature, 1050 g of 22-hydroxy-20-methylpregna-3-tetrahydropyrrole-3,5-diene was obtained by filtration and drying. The molar yield is 90.4%.
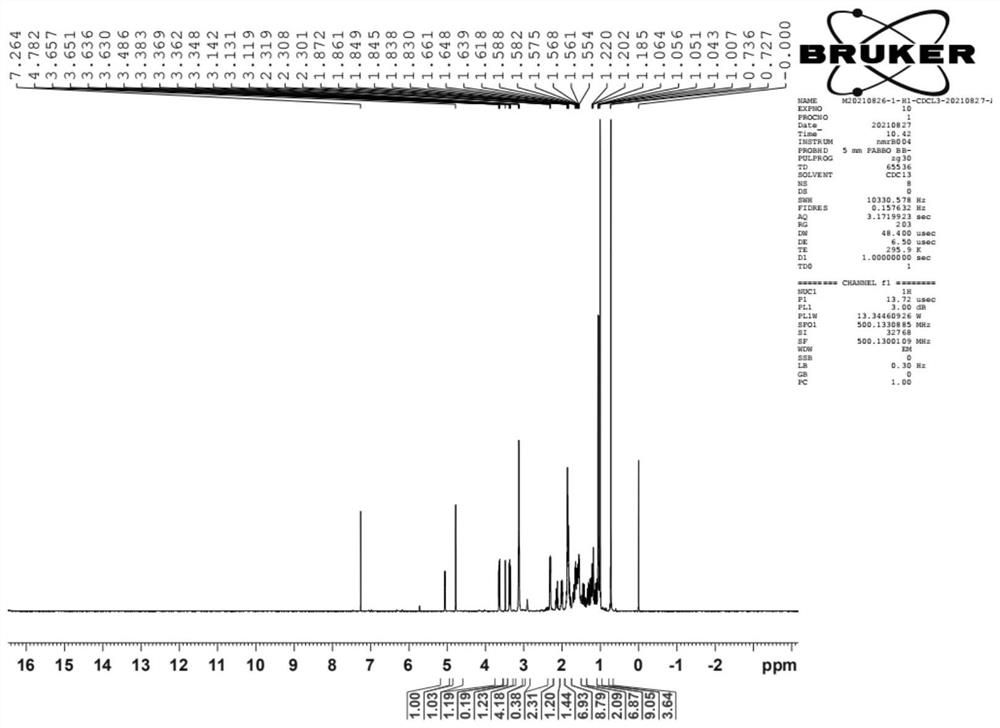
[0043] HNMR (500MHz, CDCl3) δ: 5.09(m,1H), 4.78(m,1H), 3.13(m,4H), 1.87(m,4H), 1.04(d,3H), 1.00(d,3H), 0.72(s,3H). Its spectrum is attached figure 2 shown.
[0044] 2, 3 carbonyl glycol protection
[0045] Put 1000g 22-hydroxyl-20-methylpregn-4-en-3-one into a 5000ml three-necked flask, 2000ml toluene, add 300ml ethylene glycol, 200g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com