Pancretin microparticles
A microparticle, pancreas technology, applied in the field of medicine, can solve the problems of lack of stability of enzymes, unsafe patients, health threats, etc., and achieve the effects of high solubility, loss of storage stability, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
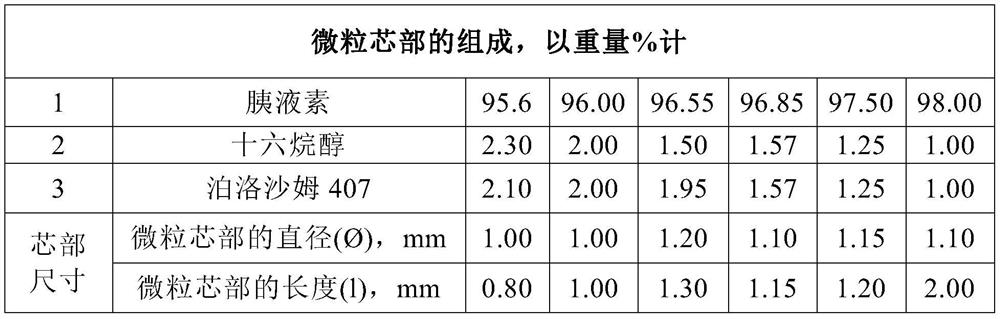
[0043] Embodiment 1 (according to the present invention)
[0044] The examples illustrate the preparation of a pharmaceutical composition comprising pancreatin in the presence of an ethanol solvent, in which microparticle cores are obtained.
[0045] First, a solution of the binder is prepared.
[0046] According to one embodiment of the present invention, ethanol is injected at 40°C to 45°C into a fabrication vessel equipped with a stirrer and a heating jacket. Then, under stirring, cetyl alcohol (powder) and poloxamer 407 (powder) were added at a ratio of ethanol: cetyl alcohol: poloxamer 407 of 1:0.11:0.11. A ternary mixture of binders is thus obtained.
[0047] According to one embodiment of the present invention, ethanol is injected at 40°C to 45°C into a fabrication vessel equipped with a stirrer and a heating jacket. Then, under stirring, cetyl alcohol (powder) and poloxamer 407 (powder) were added at a ratio of ethanol: cetyl alcohol: poloxamer 407 of 1:0.12:0.12, r...
Embodiment 2
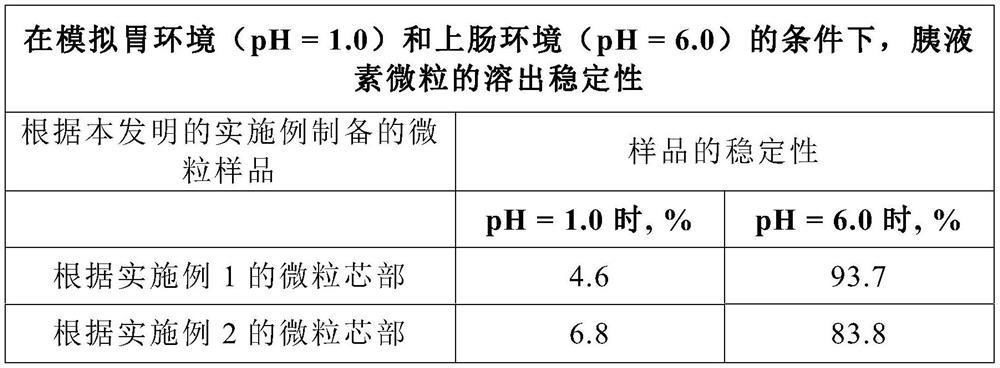
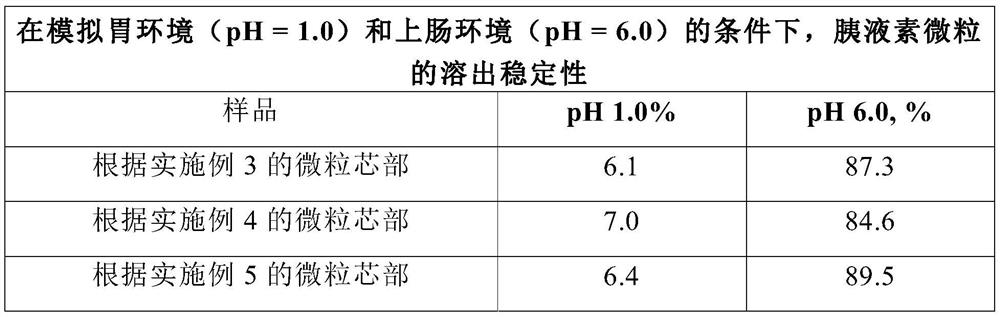
[0057] Example 2 (comparison by method of manufacturing particle cores)
[0058] The method for making the core of pancreatic insulin microparticles is similar to that of Example 1, except that, first, ethanol is charged into a mixing granulator, and pancreatin is added in batches, and then added in the ratio described in Example 1 adhesive. The mixture was mixed thoroughly for 15 minutes after each supply of one component.
Embodiment 3
[0059] Example 3 (comparison by method of manufacturing particle cores)
[0060] The method for manufacturing the core of pancreatic insulin microparticles is similar to that of Example 1, except that the prepared mixture of pancreatic insulin and binder is charged into the hopper of the molding machine. Using a mold with a hole diameter of 1.0 mm, the suspension was granulated under the condition that the drying temperature of the core was controlled at 28°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com