Hyaluronic acid oral liquid as well as preparation method and application thereof
A technology of hyaluronic acid and sodium hyaluronate, applied in the application, protein-containing food ingredients, functions of food ingredients, etc., can solve the problems of limited effect, immature formula and preparation process, etc., and achieve enhanced water retention capacity and large market. Value, the effect of solving protection problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
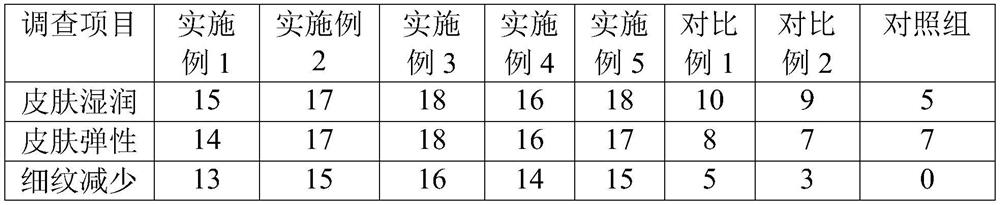
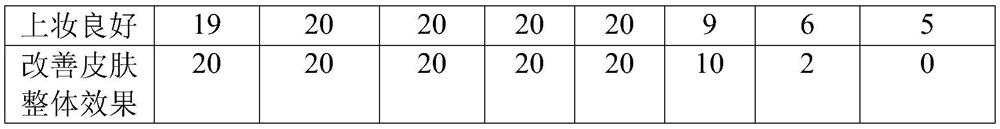
[0103] An oral solution of hyaluronic acid, which comprises the following components: sodium hyaluronate 0.6%; zinc hyaluronate 0.01%; collagen peptide 0.3%; vitamin C 0.6%; vitamin E 1%; grape seed extract 0.2%; isoflavone 0.01%; niacinamide 0.05%; potassium sorbate 0.02%; water 84%; wherein, the percentage is the percentage of the mass of each component in the total mass of the oral solution of hyaluronic acid. Among them, the molecular weight of sodium hyaluronate and zinc hyaluronate is 10kDa-70kDa, the pH value is 6.5-7.0, and potassium sorbate is selected for food grade, which is safer to eat.
[0104] The preparation method of the hyaluronic acid oral solution comprises the following steps:
[0105] Step 1: Under the condition of maintaining room temperature, combine sodium hyaluronate, zinc hyaluronate, collagen peptides, vitamin C, vitamin E, grape seed extract, isoflavones, niacinamide and potassium sorbate, slowly add to 35 °C water according to the above proportions an...
Embodiment 2
[0109] A hyaluronic acid oral solution, which comprises the following components: sodium hyaluronate 0.9%; zinc hyaluronate 0.02%; collagen peptide 0.5%; vitamin C 0.3%; vitamin E 1.2%; grape seed extract 0.5%; isoflavone 0.03%; niacinamide 0.05%; potassium sorbate 0.02%; water 85%; wherein, the percentage is the percentage of the mass of each component in the total mass of the hyaluronic acid oral solution. Among them, the molecular weight of sodium hyaluronate and zinc hyaluronate is 10kDa-70kDa, the pH value is 6.5-7.0, and potassium sorbate is selected for food grade, which is safer to eat.
[0110] The preparation method of the hyaluronic acid oral solution comprises the following steps:
[0111] Step 1: Under the condition of maintaining room temperature, combine sodium hyaluronate, zinc hyaluronate, collagen peptides, vitamin C, vitamin E, grape seed extract, isoflavones, niacinamide and potassium sorbate, slowly add to 35 °C water according to the above proportions and mix...
Embodiment 3
[0115] An oral solution of hyaluronic acid, which comprises the following components: sodium hyaluronate 1.0%; zinc hyaluronate 0.03%; collagen peptide 0.6%; vitamin C 0.8%; vitamin E 1.4%; grape seed extract 0.7%; isoflavone 0.04%; niacinamide 0.06%; potassium sorbate 0.03%; water 86%; wherein, the percentage is the percentage of the mass of each component in the total mass of the hyaluronic acid oral solution. Among them, the molecular weight of sodium hyaluronate and zinc hyaluronate is 10kDa-70kDa, the pH value is 6.5-7.0, and potassium sorbate is selected for food grade, which is safer to eat.
[0116] The preparation method of the hyaluronic acid oral solution comprises the following steps:
[0117] Step 1: Under the condition of maintaining room temperature, combine sodium hyaluronate, zinc hyaluronate, collagen peptides, vitamin C, vitamin E, grape seed extract, isoflavones, niacinamide and potassium sorbate, slowly add to 40 °C water according to the above ratio and mix w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com