Method for synthesizing triazole antifungal agent intermediate
An antifungal agent, triazole technology, applied in the field of chemical synthesis, can solve the problems of unsatisfactory product enantioselectivity, long synthetic route, poor leaving ability, etc., and achieve easy industrial expansion synthesis, mild reaction conditions, and reliable good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
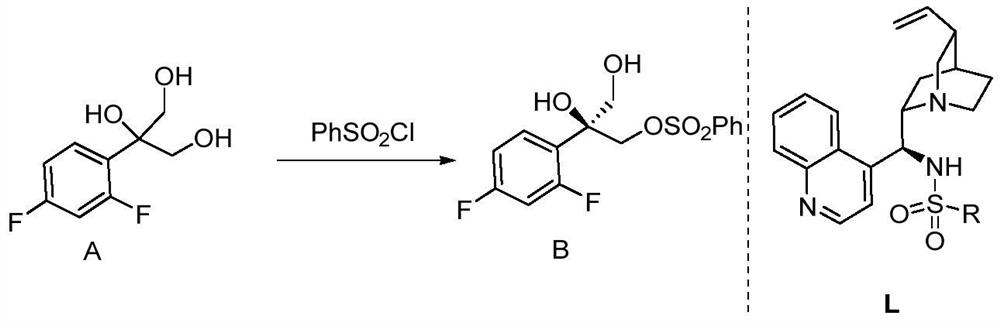
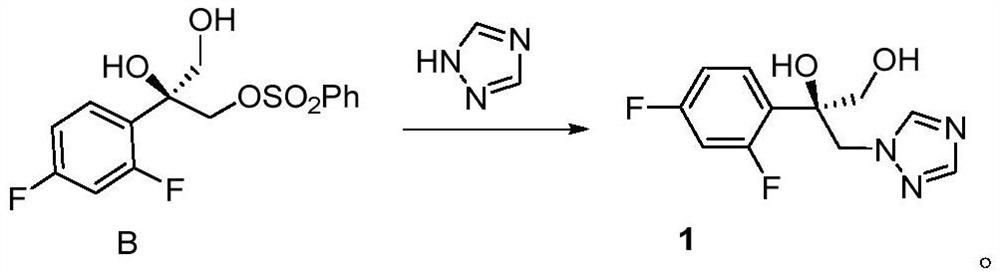
[0041] Into an oven-dried 10 mL round bottom flask equipped with a stir bar, was charged triol substrate (61.3 mg, 0.3 mmol, 1.0 eq.), cuprous iodide (5.7 mg, 0.03 mmol, 10 mol%), chiral ligand Body L1 (15.1 mg, 0.03 mmol, 10 mol%), silver carbonate (49.6 mg, 0.18 mmol, 0.6 eq.), proton sponge (12.8 mg, 0.06 mmol, 0.2 eq.), and then dry chloroform (3 mL) was added. After stirring at room temperature for 5 minutes, benzenesulfonyl chloride (46 μL, 0.36 mmol, 1.2 eq.) was added. React at room temperature for 1 day, filter with a filter device covered with diatomaceous earth, concentrate the filtrate and dissolve it with acetonitrile (3mL), then add potassium carbonate (82.8mg, 0.6mmol, 2.0eq.) and 1,2,4- Triazole (41.4mg, 0.6mmol, 2.0eq.) was reacted at 60°C for 1 day, the solvent was removed in a rotary evaporator and purified by silica gel column chromatography (petroleum ether / ethyl acetate=1 / 2). A white solid (56.5 mg, yield: 74%, ee: 93%) was obtained.
[0042...
Embodiment 2
[0050]
[0051] A 10 mL round-bottomed flask equipped with a stirring bar and oven-dried was charged with triol substrate (0.3 mmol), cuprous iodide (0.009 mmol, 3 mol%), chiral ligand L2 (0.006 mmol, 2 mol%), Silver carbonate (0.09 mmol), proton sponge (0.020 mmol) and then dry chloroform (3 mL) were added. After stirring at room temperature for 5 minutes, benzenesulfonyl chloride (0.9 mmol, 3 eq.) was added. React at room temperature for 1 day, filter with a filter device covered with diatomaceous earth, concentrate the filtrate and dissolve it with acetonitrile (3mL), then add potassium carbonate (0.9mmol, 3eq.) and 1,2,4-triazole ( 0.9mmol, 3eq.), reacted at 60°C for 1 day, removed the solvent in a rotary evaporator and purified by silica gel column chromatography (petroleum ether / ethyl acetate=1 / 2) to obtain the product (yield: 73%, ee: 83%).
Embodiment 3
[0053]
[0054] Into an oven-dried 10 mL round-bottomed flask equipped with a stirring bar, charge triol substrate (0.3 mmol), cuprous iodide (0.09 mmol, 30 mol%), chiral ligand L3 (0.045 mmol, 15 mol%), Silver carbonate (0.1 mmol), proton sponge (0.10 mmol), and then dry chloroform (4 mL) were added. After stirring at room temperature for 5 minutes, benzenesulfonyl chloride (0.6 mmol, 2 eq.) was added. React at room temperature for 1 day, filter with a filter device covered with diatomaceous earth, concentrate the filtrate and dissolve it with acetonitrile (3mL), then add potassium carbonate (0.3mmol, 1eq.) and 1,2,4-triazole ( 0.3mmol, 1eq.), reacted at 70°C for 1 day, removed the solvent in a rotary evaporator and purified by silica gel column chromatography (petroleum ether / ethyl acetate=1 / 2) to obtain the product (yield: 70%, ee: 84%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com