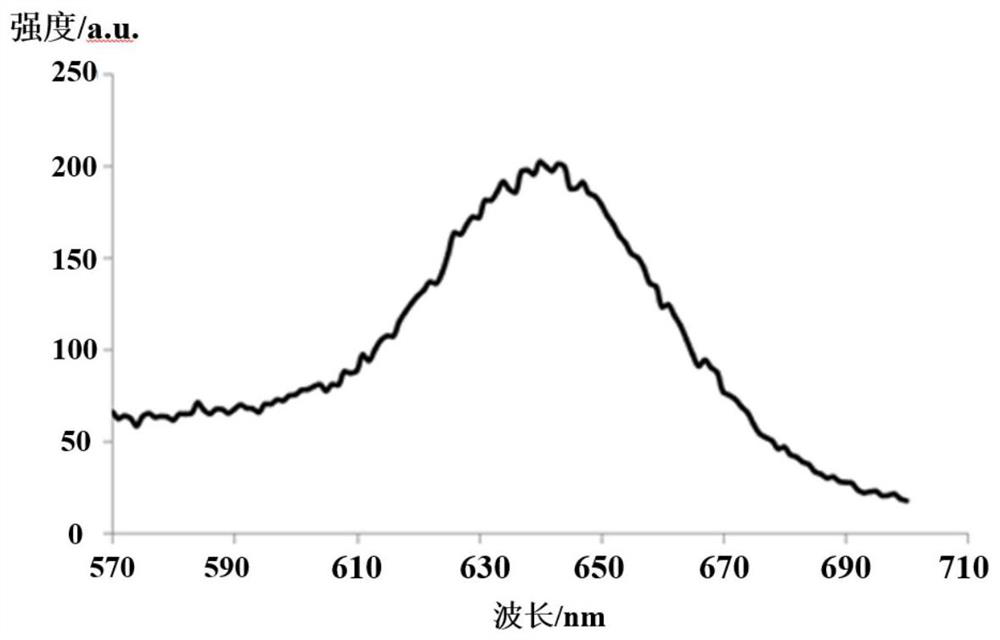
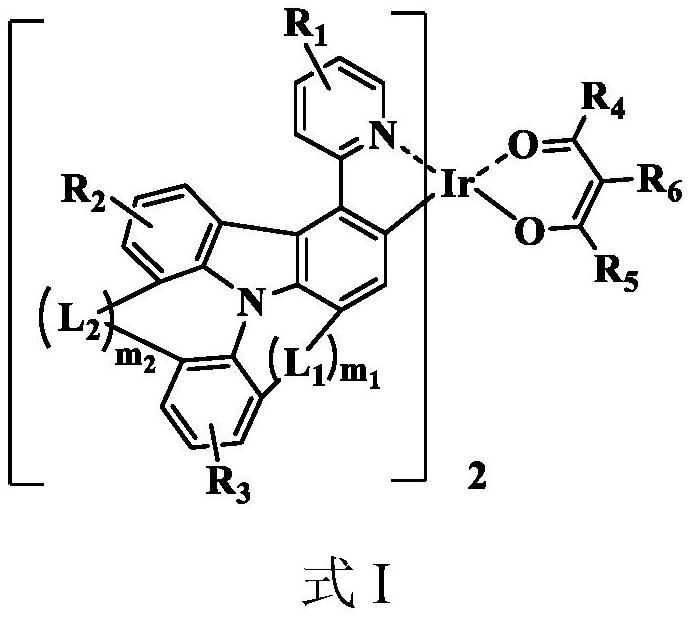
Organic metal iridium complex, electroluminescent material and application thereof
A technology of organometallic and iridium complexes, applied in the fields of luminescent materials, organic chemistry, compounds containing elements of Group 8/9/10/18 of the periodic table, etc. Red shift and other problems, to achieve the effect of improving luminescence performance, improving ligand coplanarity, and increasing coplanarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] This embodiment provides an organometallic iridium complex II-1, the structural formula of which is as follows:
[0118]
[0119] The preparation method of this organometallic iridium complex II-1 comprises the following steps:
[0120] (1) Ligand synthesis
[0121]
[0122] Synthesis of Tm-2: Add Tm-1 (49.0g, 0.2mol), pinacol ester (76g, 0.3mol), KOAc (40g, 0.4mol), Pd 2 (dba) 3 (33.7g, 4mmol), S-Phos (3.3g, 8mmol) and dioxane (600mL), N 2 Protected, 110°C reflux reaction for 16h, cooled and then spin-dried, DCM was dissolved and mixed with silica gel powder, passed through a short column, concentrated, beaten with PE, and filtered to obtain Tm-2 as a yellow solid (42.0 g, 55% yield). Mass spectrometry detects m / e=293, theoretical molecular formula C 18 h 20 BNO 2 It is 293.17, and the HPLC purity test is 96%.
[0123] Synthesis of Tm-3: Add Tm-2 (42g, 0.14mol), o-bromopyridine (22g, 0.14mol), K 2 CO 3 (39g, 0.28mol), Pd (pph 3 ) 4 (43.2g, 2.8mmol), to...
Embodiment 2
[0131] This embodiment provides an organometallic iridium complex III-1, the structural formula of which is as follows:
[0132]
[0133] The preparation method of this organometallic iridium complex III-1 comprises the following steps:
[0134] (1) Ligand synthesis
[0135]
[0136] Synthesis of Tm-6: Add Tm-3 (16g, 65mmol), o-fluorobromobenzene (23g, 0.13mol), Cs 2 CO 3 (43g, 0.13mol) and DMF (180mL), 150°C under reflux for 16h in nitrogen, washed with water after cooling, extracted with DCM, dried over anhydrous sodium sulfate, purified by silica gel column and recrystallized from toluene to obtain Tm-6 as a white solid (6.6 g, 25% yield). Mass spectrometry m / e=399, theoretical molecular formula C 23 h 15 BrN 2 It is 398.29, and the HPLC purity test is 99%.
[0137] Synthesis of Tm-7: Add Tm-6 (7.4g, 18.6mmol), K 2 CO 3(5.2g, 37.2mmol), Pd(OAc) 2 (0.084g, 372mmo), t-Bu 3 PF 4 B (0.22g, 0.744mmol) and DMAc (50mL), N 2 Reflux reaction at 180°C for 16h under...
Embodiment 3
[0145] This embodiment provides an organometallic iridium complex IV-10, the structural formula of which is as follows:
[0146]
[0147] The preparation method of this organometallic complex IV-10 comprises the following steps:
[0148] (1) Ligand synthesis
[0149]
[0150] Synthesis of Tm-10: Add Tm-9 (42g, 150mmol), 1-fluoro-3methoxybenzene (22g, 180mol), Cs 2 CO 3 (97g, 0.3mol) and DMF (300mL), reflux reaction at 150°C in nitrogen for 16h, cooled, washed with water, extracted with DCM, dried over anhydrous sodium sulfate, purified by silica gel column and recrystallized from toluene to obtain Tm-10 as a white solid (46.2g, 80% yield); Mass spectrometry detects m / e=386, theoretical molecular formula C 19 h 13 BrClNO is 386.67, and the HPLC purity test is 99%.
[0151] Synthesis of Tm-11: Add Tm-10 (38g, 0.1mol), 4-deuteropicoline-2 borate (22g, 0.1mol), K 2 CO 3 (27.6g, 0.2mol), Pd (pph 3 ) 4 (1g, 1mmol), toluene (200mL), ethanol (70mL) and water (100mL), N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com