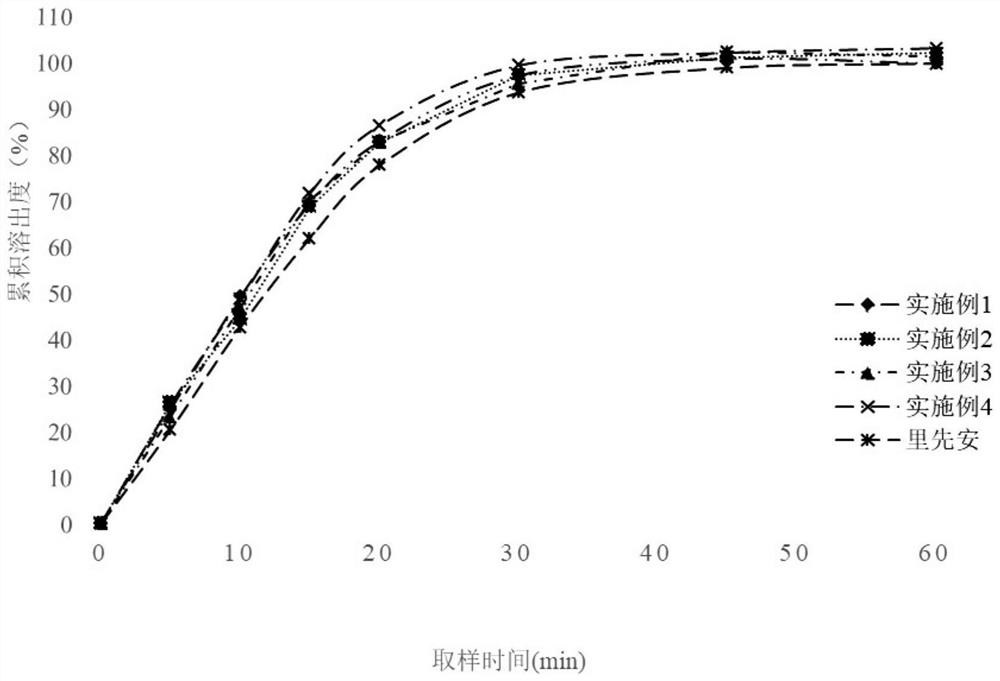
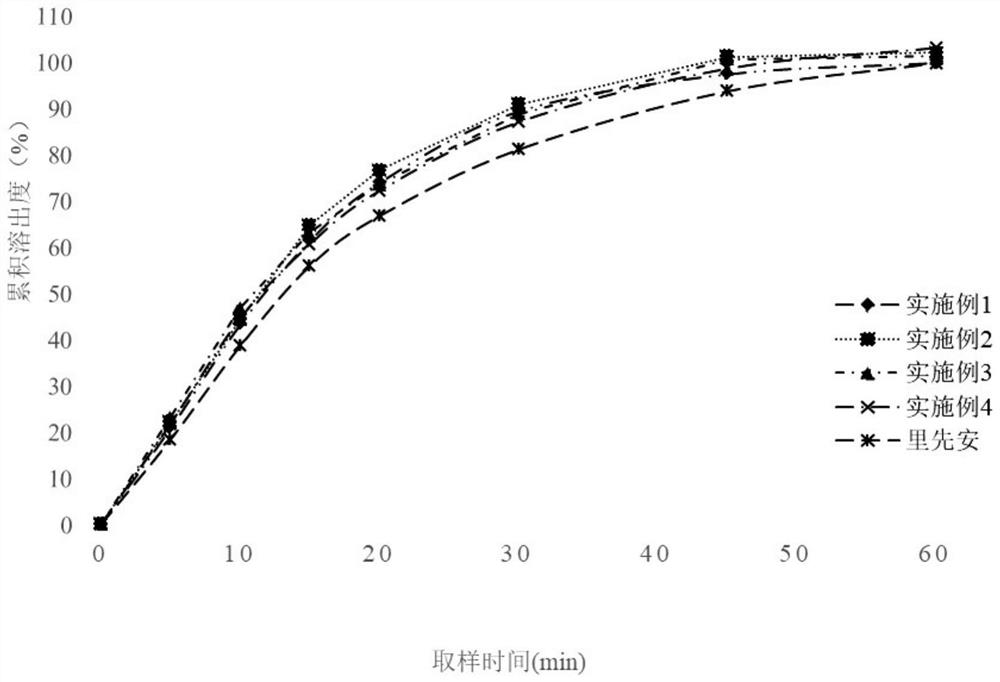
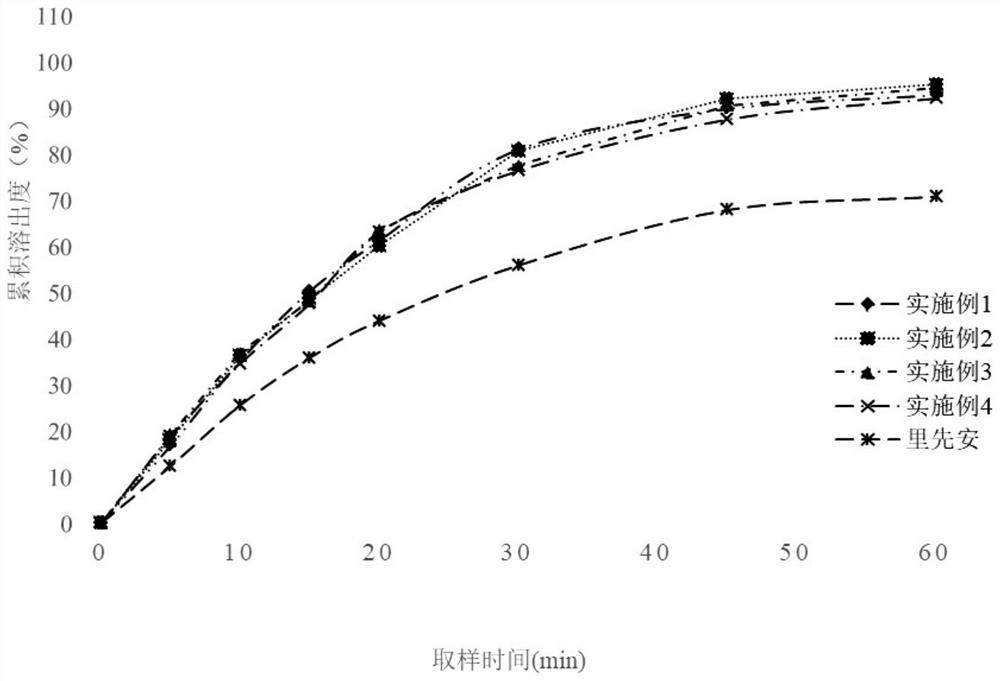
Edoxaban tosylate preparation and preparation method thereof
A technology of edoxaban and toluenesulfonic acid, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of reduced bioavailability, affecting drug absorption, and adverse clinical treatment and other problems, to achieve the effect of improving the dissolution rate and degree of dissolution, low technical difficulty, and reducing the degree of crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention also provides a preparation method of edoxaban tosylate preparation, comprising the following steps:
[0034] 1) Weighing 20-30% of edoxaban tosylate and 70-80% of hydrophilic carrier material by mass percentage, adding purified water 1.5-4 times the total weight of raw materials to prepare a suspension;
[0035] 2) Nano-grind the above suspension, the grinding speed is 1500rpm-2400rpm, the grinding temperature is 4°C-40°C, and the grinding is stopped until the volume average particle size of the active ingredient reaches 200-400nm;
[0036] 3) Spray-dry the suspension obtained by grinding, enter the air at 118°C-128°C, control the spray speed, keep the outlet air temperature at 55°C-65°C, and maintain a negative pressure of 300Pa-500Pa, and prepare the volume average particle Diameter 125μm-850μm, BET surface area 0.5-2m 2 / g of loose porous microspherical solid dispersion;
[0037] 4) The solid dispersion is prepared by conventional methods to ...
Embodiment 1
[0040] The preparation of embodiment 1 edoxaban tosylate capsules
[0041] Edoxaban tosylate preparation 1, the solid dispersion contains 10% of edoxaban tosylate and 90% of hydrophilic carrier material (by mass percentage).
[0042]
[0043] The specific preparation method is as follows:
[0044] (1) Add edoxaban tosylate and mannitol into purified water whose total weight is 1.5 times that of raw materials to prepare a suspension.
[0045] (2) Nanogrind the above suspension (the grinding medium is zirconia beads with an average particle diameter of 0.3 mm), set the grinding temperature at 10° C., rotate at 2000 rpm, and grind for 60 minutes to obtain a mixture of raw materials with a volume average particle diameter of 200 nm. Suspension.
[0046] (3) Spray-dry the ground suspension to prepare a loose porous microspherical solid dispersion.
[0047] Set the inlet air temperature to 120°C, the liquid spray speed to 300g / min, the outlet air temperature to 55°C, and maint...
Embodiment 2
[0049] The preparation of embodiment 2 edoxaban tosylate tablets
[0050] Edoxaban tosylate preparation 2, the solid dispersion contains 20% of edoxaban tosylate and 80% of hydrophilic carrier material (by mass percentage).
[0051]
[0052] The specific preparation method is as follows:
[0053] (1) Add edoxaban tosylate and galactose into purified water with 2.5 times the total weight of raw materials to prepare a suspension.
[0054] (2) Nanogrind the above suspension (the grinding medium is zirconia beads with an average particle diameter of 0.3mm), set the grinding temperature at 4°C, the rotation speed at 2400rpm, and the grinding time for 50min to obtain a mixture of raw materials with a volume average particle diameter of 300nm. Suspension.
[0055] (3) Spray drying the ground suspension to prepare a loose porous microspherical solid dispersion;
[0056] Set the inlet air temperature to 118°C, the liquid spray speed to 400g / min, the outlet air temperature to 60°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com