ROS-responsive controlled-release ophthalmic preparation and preparation method thereof
A technology of ophthalmic preparations and responsive groups, applied in the field of biomedicine, can solve problems such as eye trauma, long-term wearing, drug side effects, etc., and achieve the effect of improving utilization, avoiding side effects, and safe and effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] In the present invention, the preparation method of the ROS-responsive nano drug carrier preferably includes the following steps: mixing the carboxyl ROS-responsive group, ethylene glycol chitosan aqueous solution and a coupling agent, performing amidation reaction, dialysis, The intercepted product of the dialysis is dried to obtain a ROS-responsive nano drug carrier.
[0030] In the present invention, the solvent of the carboxyl ROS responsive group is preferably methanol; the concentration of the biodegradable carbohydrate compound in the biodegradable carbohydrate compound solution is preferably 5-20 μg / mL; more preferably 13.59 μg / mL.
[0031] In the present invention, the concentration of ethylene glycol chitosan in the ethylene glycol chitosan aqueous solution is preferably 0.01-1 μg / mL; more preferably 0.417 μg / mL.
[0032] In the present invention, the coupling agent is preferably N-hydroxysuccinimide (NHS) and (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide h...
Embodiment 1
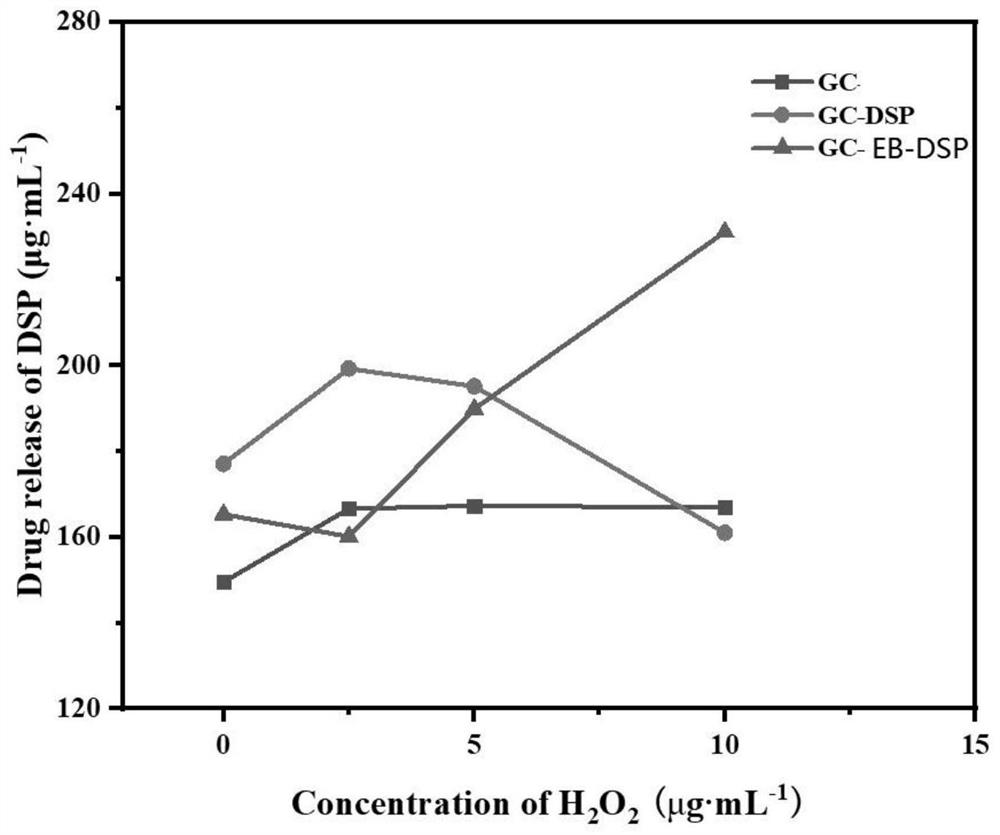
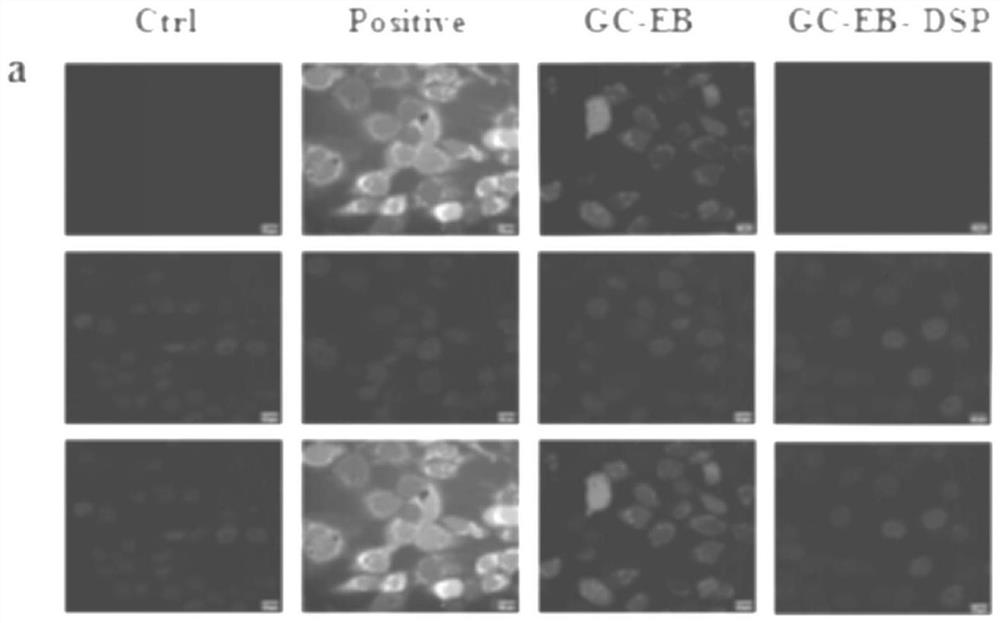
[0046] Dissolve 0.816g 4-carboxyphenylboronic acid pinacol ester (EB) in 45mL methanol, add 0.025mg / mL ethylene glycol chitosan (GC) aqueous solution to 15mL, stir well, add 0.563g N-hydroxysuccinyl Imine (NHS) and 0.761g (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) (EDC), heated to 70°C, and kept stirring for 24h. After three days of dialysis, EB-modified GC-EB was obtained by freeze-drying.
[0047] Characterized by infrared spectroscopy at 1725cm -1 A new boronic ester C=O bond appeared after the modification of phenylboronic acid, and at 714cm -1 At the B-O bond, at 1544 and 902cm -1 The stretching vibration peak of the C=C bond of the benzene ring is at the peak, which proves that the modification of phenylboronic acid for ROS-responsive molecules is successful.
Embodiment 2
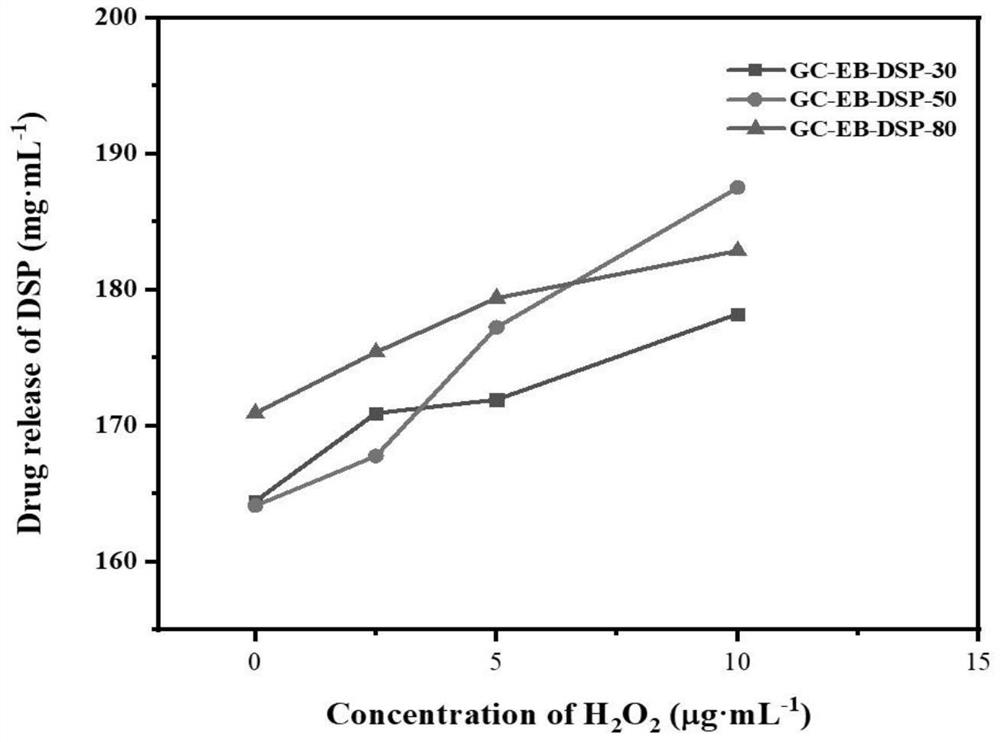
[0055] Dissolve 0.01 g of GC-EB prepared in Example 1 in 15 mL of distilled water, ultrasonicate for 15 min, add 0.030 g of dexamethasone sodium phosphate (DSP) into the above solution system, and continue stirring for 24 h. GC-EB-DSP ROS-responsive nano-drug carriers were prepared by dialysis and freeze-drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Mwco | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com