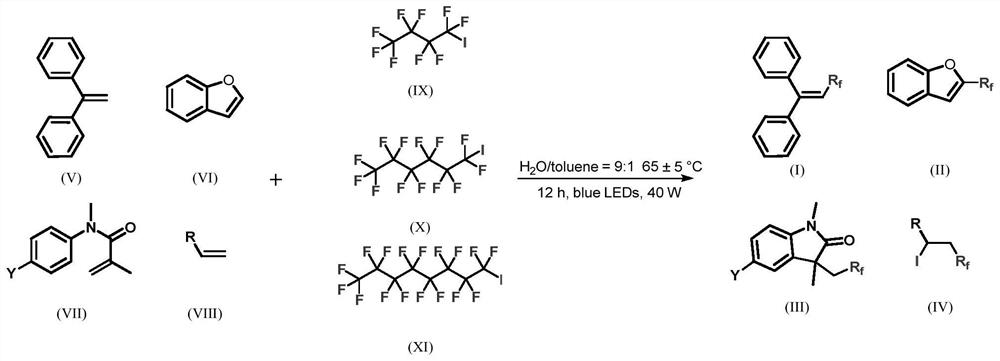
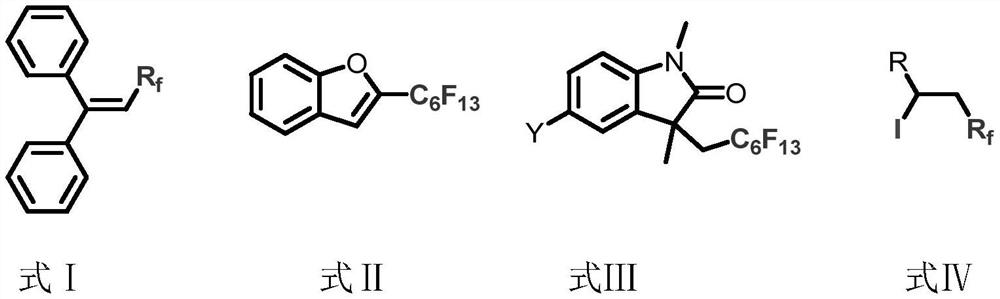
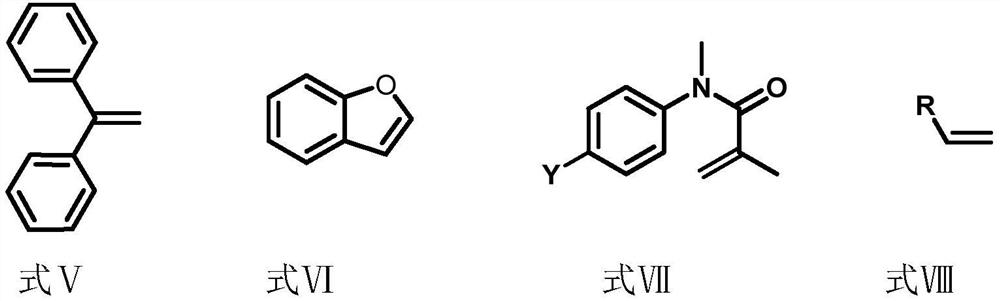
Synthetic method of perfluoroalkane compound and derivative thereof
A synthesis method and technology of perfluoroalkane iodide, which is applied in the field of synthesis of perfluoroalkane compounds and their derivatives, can solve the problems of economy and environmental protection, and achieve mild reaction conditions, simple operation, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, compound shown in synthetic formula II-1
[0034] according to figure 1 The compound shown in the synthetic route diagram synthetic formula II-1, concrete steps are as follows:
[0035] The pre-dried reaction tube was lowered to room temperature under vacuum, and the compound represented by formula VI (0.2 mmol) and perfluoroiodohexane represented by formula X (0.4 mmol) were added under the protection of nitrogen. After adding toluene (0.05 mL) and water (0.45 mL) to the mixture via syringe. Stir overnight (12h) at 60°C under a 50W blue LED (460nm) lamp. Then the reaction mixture is distilled under reduced pressure, separated by silica gel chromatography (the stationary phase of column chromatography: SiO 2 , mobile phase: petroleum ether, after collection, vacuum distillation obtains the product), to obtain the compound shown in formula II.
[0036]
[0037] The experimental data for structural verification are as follows:
[0038] Transparent oi...
Embodiment 2
[0040] Embodiment 2, compound shown in synthetic formula III-1
[0041] according to figure 1 The compound shown in the synthetic route diagram synthetic formula III-1, concrete steps are as follows:
[0042] The pre-dried reaction tube was reduced to room temperature under vacuum, and the compound represented by formula VII-1 (0.2 mmol) and perfluoroiodohexane represented by formula X (0.4 mmol) were added under nitrogen protection. After adding toluene (0.05 mL) and water (0.45 mL) to the mixture via syringe. Stir overnight (12h) at 60°C under a 50W blue LED (460nm) lamp. The reaction mixture was then subjected to silica gel chromatography (the stationary phase of column chromatography: SiO 2 , mobile phase: petroleum ether / ethyl acetate=5:1, the product was obtained by distillation under reduced pressure after collection), to obtain the desired compound of III-1.
[0043]
[0044] The experimental data for structural verification are as follows:
[0045] white solid...
Embodiment 3
[0047] Embodiment 3, compound shown in synthetic formula III-2
[0048] according to figure 1 The compound shown in the synthetic route diagram synthetic formula III-2, concrete steps are as follows:
[0049] The pre-dried reaction tube was lowered to room temperature under vacuum, and the compound represented by formula VII-2 (0.2 mmol) and perfluoroiodohexane represented by formula X (0.4 mmol) were added under nitrogen protection. After adding toluene (0.05 mL) and water (0.45 mL) to the mixture via syringe. Stir overnight (12h) at 60°C under a 50W blue LED (460nm) lamp. The reaction mixture was then subjected to silica gel chromatography (the stationary phase of column chromatography: SiO 2 , mobile phase: petroleum ether / ethyl acetate=5:1, the product was obtained by distillation under reduced pressure after collection), to obtain the desired compound of III-2.
[0050]
[0051] The experimental data for structural verification are as follows:
[0052] white solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com