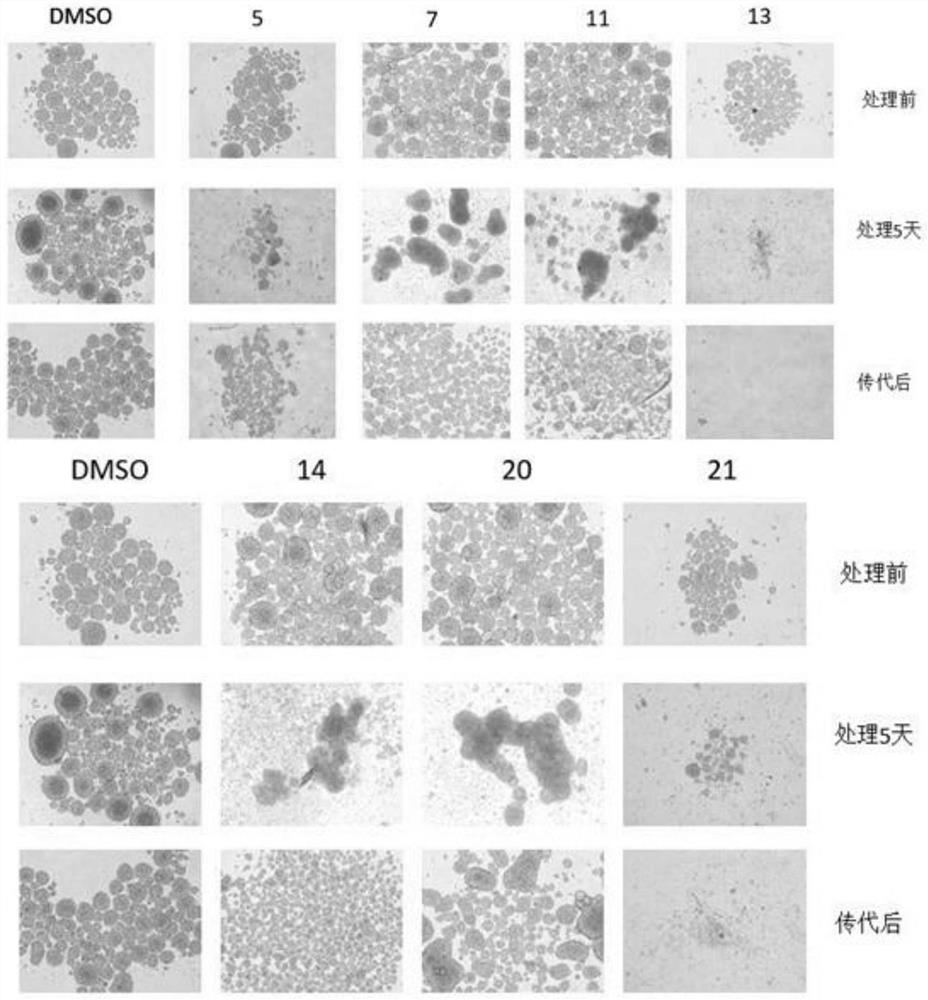
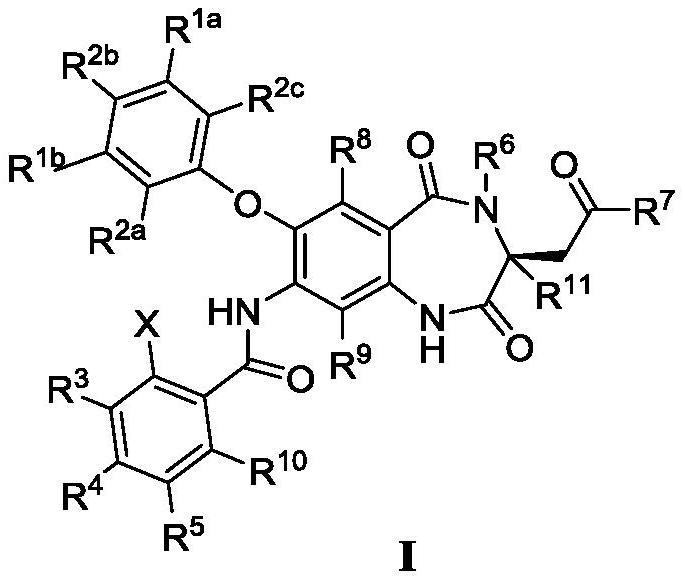
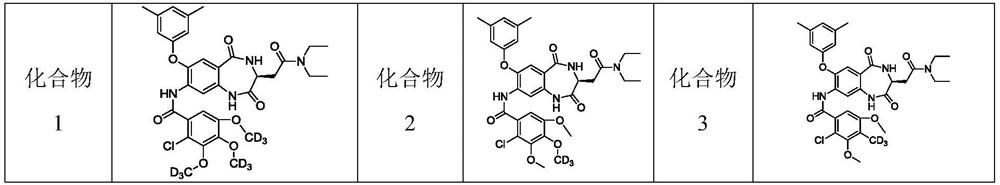
Deuterated 1, 4-benzodiazepine-2, 5-diketone compound and application thereof
A compound and deuterium technology, which is applied in the field of deuterated 1,4-benzodiazepine-2,5-dione compounds, can solve the problems of short half-life and achieve extended half-life, good inhibitory activity, and extended The effect of degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0300] Example 1 (S)-2-Chloro-N-[3-[2-(diethylamino)-2-oxoethyl]-7-(3,5-dimethylphenoxy)-2,5- Dioxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-8-yl]-3,4,5-tris(trideuteromethoxy base) benzamide (compound 1)
[0301] Preparation of 5-chloro-2,4-dinitrobenzoic acid (intermediate 42): Dissolve 3-chlorobenzoic acid (63.9 mmol) in 120 mL of concentrated sulfuric acid with stirring at room temperature, and add potassium nitrate (163.2 mmol). The reaction solution was reacted at 80° C. for 30 minutes, 110° C. for 2 hours, and 120° C. for 2 hours. The reaction solution was poured into 660 g of ice water, filtered, and the resulting white solid was recrystallized with a mixed solvent of ethanol and water to obtain 8.08 g of light yellow crystals, with a yield of 51.3%. 1 H NMR (400MHz, DMSO-d 6 )δ14.21(brs,1H),8.85(s,1H),8.28(s,1H). 13 C NMR (100MHz, DMSO-d 6 )δ163.78, 147.95, 145.70, 132.86, 132.06, 130.52, 122.05.
[0302]Preparation of 5-(3,5-dimethylphenoxy)-2,4-dinitrob...
Embodiment 2
[0308] Example 2 (S)-2-Chloro-N-[3-[2-(diethylamino)-2-oxoethyl]-7-(3,5-dimethylphenoxy)-2,5- Dioxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-8-yl]-3,5-dimethoxy-4-(tri deuterated methoxy) benzamide (compound 2)
[0309] Preparation of 2-chloro-3,5-dimethoxy-4-(trideuteromethoxy)benzoic acid (Intermediate 48): Syringic acid (2.52 mmol), potassium carbonate (10.10 mmol) were dissolved in 5 mL Add deuteroiodomethane (5.05 mmol) to DMF, and react overnight at 35°C. It was detected that a small amount of substrate was not completely reacted, and deuterated methyl iodide (5.05 mmol) was added, and the reaction was continued for 9 h, and the reaction was completed. The reaction solution was evaporated to dryness, dissolved in DCM, washed three times with water, the organic phase was collected, dried and concentrated to obtain 544 mg of intermediate product with a yield of 93.0%. The above intermediate (2.34 mmol) and NCS (2.81 mmol) were dissolved in 5 mL DMF, and reacted ov...
Embodiment 3
[0311] Example 3 (S)-2-Chloro-N-[3-[2-(diethylamino)-2-oxoethyl]-7-(3,5-dimethylphenoxy)-2,5- Dioxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine-8-yl]-3,5-dimethoxy-4-(tri Deuterated methyl) benzamide (compound 3)
[0312] Preparation of 2-chloro-3,5-dimethoxy-4-(trideuteromethyl)benzoic acid (intermediate 49): Dissolve potassium tert-butoxide (4.38 mmol) in 10 mL of anhydrous THF, stir, 10 mL of anhydrous THF solution dissolved in 3,5-dimethylbenzoic acid (1.10 mmol) was added under the protection of argon at -78°C. Add butyllithium (4.38mmol, 2.5M in hexanes), and react at -78°C for 40min. Deuteroiodomethane (2.20 mmol) was added, and the reaction was continued for 1 h at -78°C. The reaction solution was raised to room temperature, quenched by adding 6 mL of saturated ammonium chloride solution, washed twice with 10 mL of ether, and the water phase was separated, and the pH of the water phase was adjusted to acidic with 2M hydrochloric acid, and a white precipitate was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com