Sorbose reductase OpCR gene, mutant, encoding protein and application in preparation of glassine
A reductase and sorbose technology, applied in oxidoreductase, application, genetic engineering and other directions, can solve the problems of difficulty in industrial scale-up and complicated operation, and achieve the effect of reducing production costs and overcoming difficulties in process scale-up.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The influence of embodiment 1 different catalysts on the preparation reaction of β-acetone xyloside
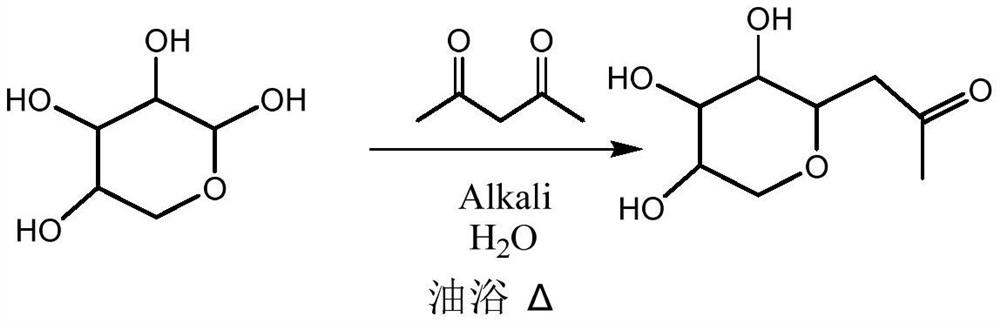
[0029] Xylose (10g) was completely dissolved in deionized water (40mL), added to a 100mL three-necked flask, added different catalysts (1.25eq), and reacted at 90°C for 18 hours. The chemical reaction formula is as follows, and the reaction results are shown in Table 1 shown.
[0030]
[0031] The influence of table 1 catalyst on the preparation of β-acetone xyloside
[0032] alkali Reaction temperature °C Response time h Yield% NaHCO 3
90 18h 87% LiOH 90 18h 56% NaOH 90 18h 88%
[0033] According to the results in Table 1, it can be seen that under the above-mentioned catalysts, the method of the present invention can obtain higher yields. Taken together, the catalysts are NaOH and NaHCO 3 The response is better.
[0034] The influence of embodiment 1 reaction temperature and time on the preparation reaction of β-a...
Embodiment 3
[0040] The preparation of embodiment 3 boson
[0041] 1. Preparation of β-acetone xyloside
[0042] Xylose (100g) was completely dissolved in deionized water (400mL), added to a 1000mL three-necked flask, NaOH (53.2g, 2eq), and reacted at 50°C for 1 hour. The pH value of the solution is adjusted to neutral, and the solvent is removed to obtain the first intermediate product β-acetone xyloside.
[0043] 2. Cloning of β-acetone xyloside reductase gene and construction of recombinant bacteria
[0044] The enzymes required by the present invention are all synthesized by the company and then constructed on the expression plasmid and then produced by E. coli fermentation; it specifically includes the following steps:
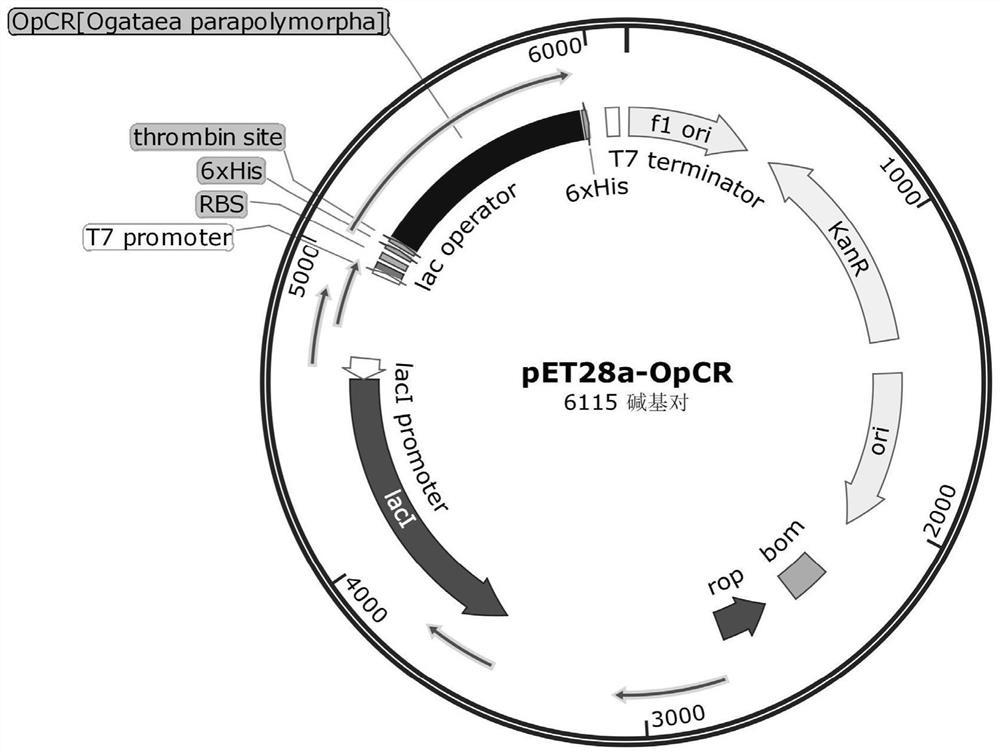
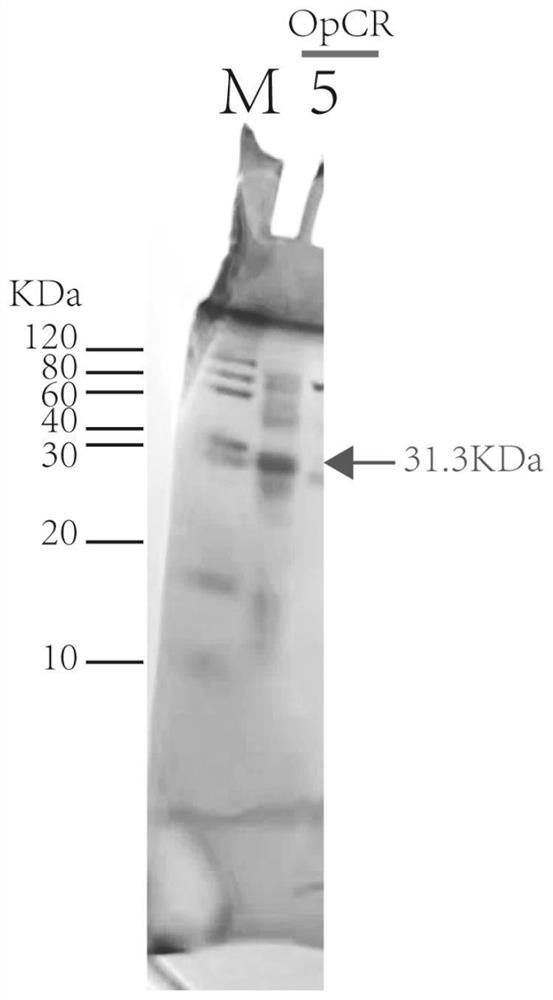
[0045] Derived from the sorbose reductase OpCR gene of Ogataea parapolymorpha, the preservation number is JCM 22074 (purchased from the Japan Microorganism Collection Center), the gene sequence is shown in SEQ ID NO.1, and the amino acid sequence is shown in SEQ ID ...
Embodiment 4
[0072] Example 4OpCR E70D / Q92L / M135F Enzymatic preparation of Bosene
[0073] 30g crude product of β-acetone xyloside was dissolved in 1.0L phosphate buffer (K 2 HPO 4 -KH 2 PO 4 ) buffer (50mM, pH 8.0) solution, then add 0.34mM, 30g glucose, 2.5g reduced β-nicotinamide adenine dinucleotide disodium tetrasodium phosphate (NADPH), and 6000U glucose dehydrogenase GDH. Add crude enzyme solution (OpCR E70D / Q92L / M135F ), stirred slowly at 30°C for 24 hours, after the reaction, centrifuged, desalted the supernatant by nanofiltration, evaporated water under reduced pressure, and purified to obtain 29.8g product with a purity of 98.9% and a yield of 98.3%.
[0074] The reaction formula is as follows:
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com