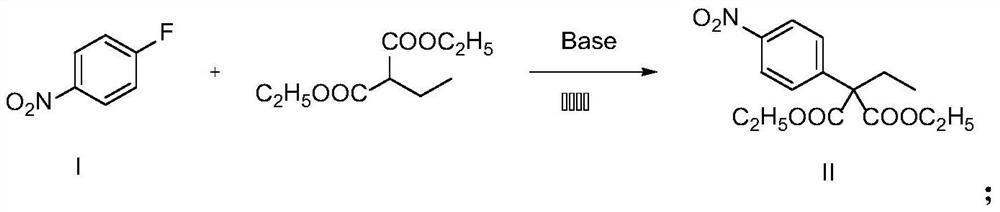
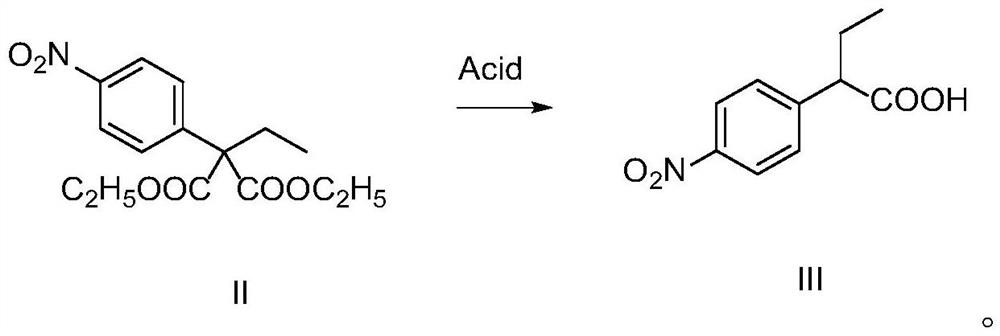
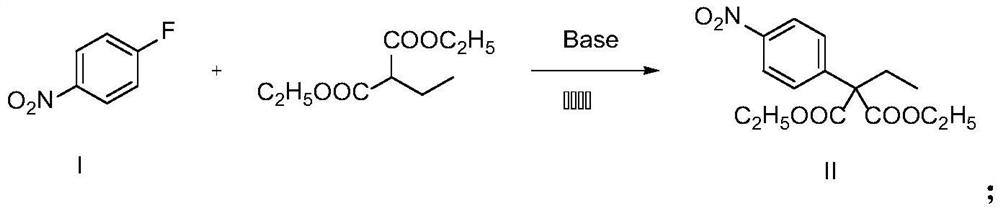
Synthesis method of 2-(4-nitrophenyl) butyric acid
A synthetic method, nitrophenyl technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as the impact of environmental impact assessment, and achieve the effect of convenient operation, less pollution, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022]In the reaction bottle, SM2 (1.41g, 10.00mmol) was dissolved in DMF, cooled to 0 °C, NaH (0.28g, 12.00mmol) was added under the ice bath, stirred for half an hour and then dripped diethyl malonate (2.83g, 15.00mmol), the ice bath was removed, and the reaction at room temperature was 12h. After the reaction was completed, the reaction solution was poured into water, extracted with ethyl acetate, combined with organic phase, washed with saturated brine, dried anhydrous sodium sulfate, and purified by column chromatography to give a light yellow oily liquid C002.10g, with a yield of 67%.
[0023] 1 H NMR(60MHz,CDCl 3 )δ8.44–8.02(m,2H),7.88–7.48(m,2H),4.53–4.08(m,4H),2.39(q,J=7.4Hz,2H),1.06(dt,J=22.6,7.3Hz,9H).
[0024] HRMS(ESI):found 310.1290[M+H] +
Embodiment 2
[0026]
[0027] Add SM2 (1.41 g, 10.00 mmol) and K to the reaction flask 2 CO 3 (4.14g, 30.00mmol), dissolved in DMF, stirred and dripped with diethyl malonate (2.83g, 15.00mmol), room temperature reaction 12h. After the reaction was completed, the reaction solution was poured into water, extracted with ethyl acetate, combined with the organic phase, washed with saturated salt water, dried anhydrous sodium sulfate, and purified by column chromatography to give a pale yellow oily liquid C00 2.00g, with a yield of 64%.
[0028] 1 H NMR(60MHz,CDCl 3 )δ8.44–8.02(m,2H),7.88–7.48(m,2H),4.53–4.08(m,4H),2.39(q,J=7.4Hz,2H),1.06(dt,J=22.6,7.3Hz,9H).
[0029] HRMS(ESI):found 310.1290[M+H] +
Embodiment 3
[0031]
[0032] In the reaction bottle, C00 (0.31g, 1.00mmol), dissolved in acetic acid, stirred and dripped 50% concentrated sulfuric acid (0.6mL, 3.00mmol), 120 °C reaction for 24h. After the reaction, the reaction liquid is dried, add water, NaOH is adjusted to alkaline, dichloromethane extraction, the organic phase is discarded, the aqueous phase is adjusted to pH to acidic with HCl, dichloromethane is extracted, the organic phase is saturated with salt water washed, the anhydrous sodium sulfate is dried, and the pale yellow solid B00 is obtained by spinning drying to obtain a pale yellow solid B00 1.50g, with a yield of 76%.
[0033] 1 H NMR(60MHz,DMSO-d 6 )δ8.48–7.97(m,2H),7.85–7.36(m,2H),3.72(d,J=7.4Hz,1H),2.28–1.59(m,2H),0.83(t,J=7.2Hz,3H).
[0034] HRMS(ESI):found 208.0615[M-H] -
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com