Naproxen oral preparation and preparation method thereof
A technology for oral preparations and excipients, applied in the field of naproxen oral preparations and preparation thereof, achieves the effects of being convenient to take, increasing the dissolution rate, and improving the prescription and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
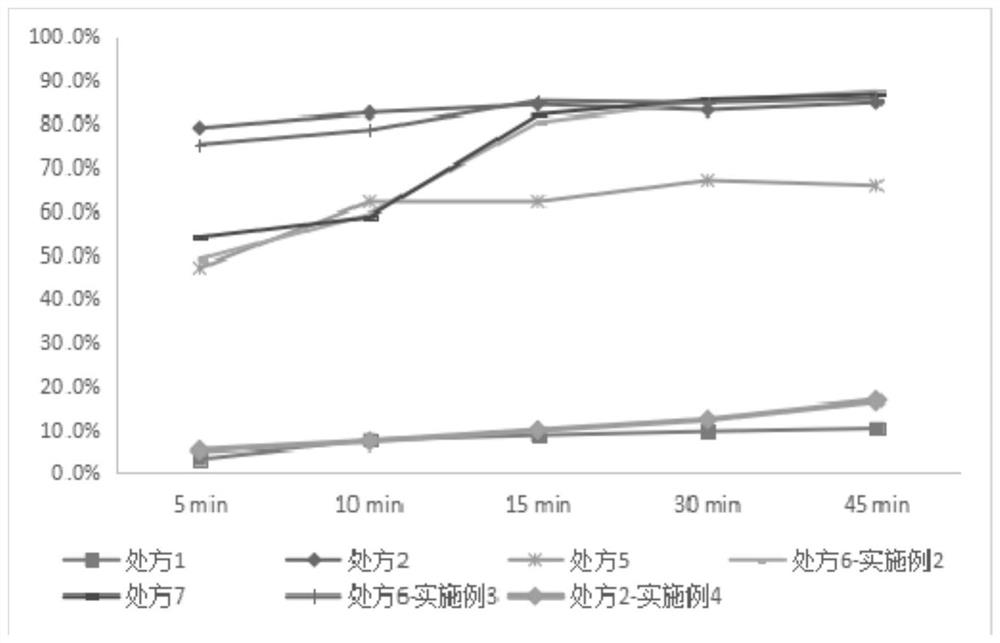
Image
Examples
Embodiment 1
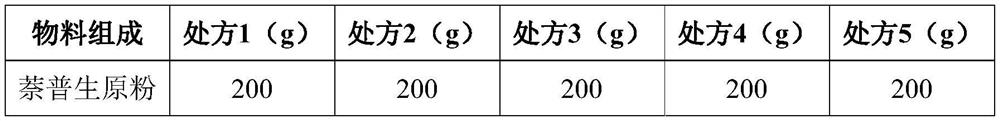
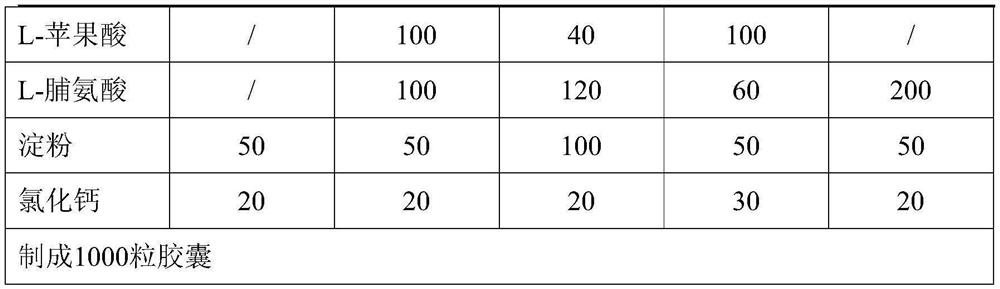
[0028] Oral formulation of naproxen:
[0029]
[0030]
[0031] The preparation method of naproxen oral preparation:
[0032] (1) Mix the original naproxen powder, malic acid and proline (if there is no such ingredient in the recipe), and grind it at 25 to 30°C for 30 to 50 minutes (preferably 45 minutes in this example), and during the grinding process Add 40-50% ethanol aqueous solution (in this case, the preferred concentration is 50%) dropwise evenly, and after grinding, let it stand for at least 1h (in this case, preferably 1h) under the condition of ≤15°C (preferably 12°C in this case), and vacuum dry; Wherein, the dosage of the ethanol aqueous solution is 0.01 to 0.02 times (preferably 0.01 times in this example) of the total mass of the original naproxen powder.
[0033] (2) mixing the material treated in step (1) with the remaining auxiliary materials uniformly, and sieving; filling the uniformly mixed material into the capsule shell.
Embodiment 2
[0035] Oral formulation of naproxen:
[0036]
[0037] The preparation method of naproxen oral preparation:
[0038] (1) Mix the original naproxen powder, malic acid and proline, and grind them at 25 to 30°C for 30 to 50 minutes (preferably 45 minutes in this example), and evenly dropwise add 40 to 50% of Aqueous ethanol solution (preferably 50% in this example), after grinding, let stand for at least 1 hour (preferably 1 hour in this example) under the condition of ≤15°C (preferably 12°C in this case) to obtain material A;
[0039] (2) at normal temperature, add starch and calcium chloride in material A, evenly drop the sodium alginate aqueous solution (preferably 1% in this example) with a concentration of 1 to 1.5% dropwise and stir and mix, and vacuum dry; wherein, the consumption of the ethanol aqueous solution is 0.01 to 0.02 times the total mass of the original naproxen powder (preferably 0.01 times in this example).
[0040] (3) Filling the material processed in s...
Embodiment 3
[0042] Take prescription 6 formula and prepare capsules as follows:
[0043] (1) Mix the raw naproxen powder, malic acid and proline and grind at 25 to 30°C for 45 minutes, and evenly add 50% ethanol aqueous solution dropwise during the grinding process, and grind at 12°C after grinding. Stand for 1 hour, and vacuum dry; wherein, the amount of the ethanol aqueous solution is 0.01 times the total mass of the original naproxen powder.
[0044] (2) Mixing the material treated in step (1) with the remaining auxiliary materials uniformly, and filling the uniformly mixed material into the capsule shell.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com