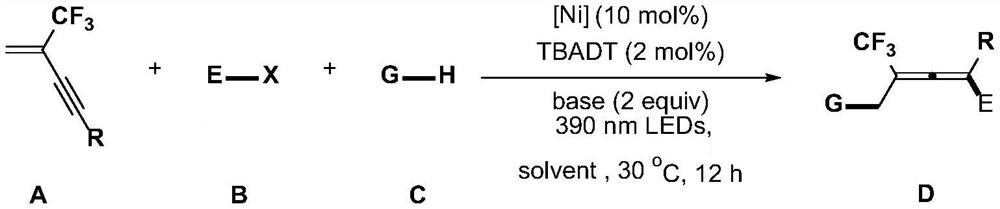
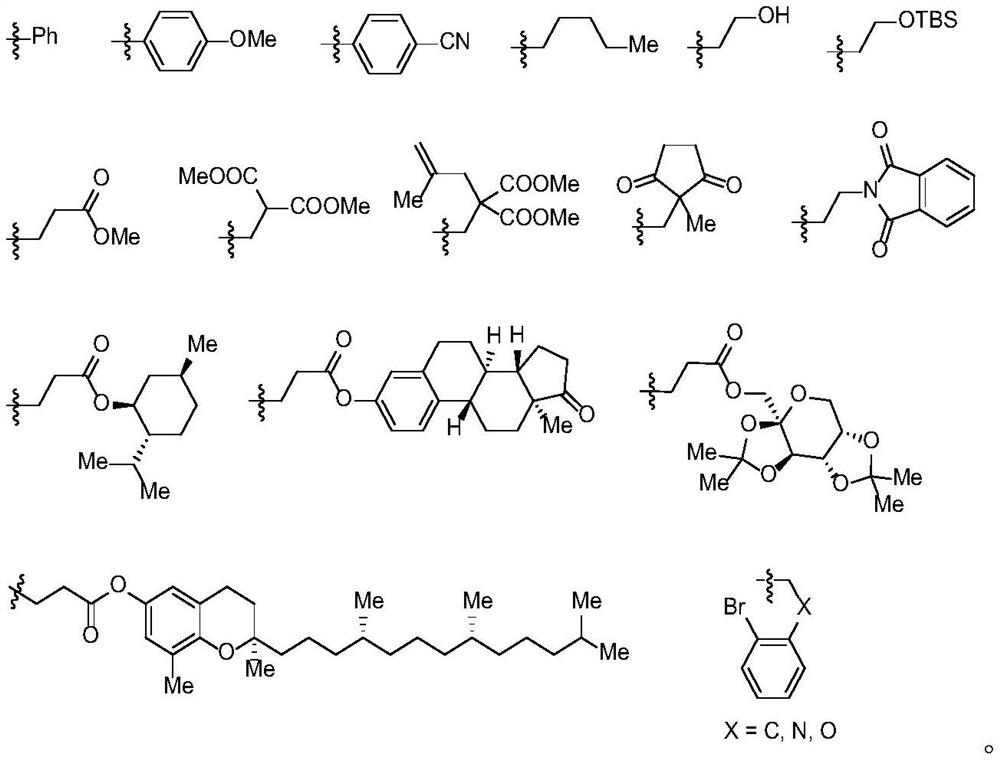
Modular synthesis method of polysubstituted trifluoromethyl allene
A technology of trifluoromethyl allene and trifluoromethyl, applied in chemical instruments and methods, preparation of organic compounds, steroids, etc., to achieve strong compatibility, low-cost synthesis, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Under an inert (argon) atmosphere, sodium decapolytungstate (13.3 mg, 0.004 mmol), (4,4'-di-tert-butyl-2,2'-bipyridine) was added to the reaction tube containing the magnetron. Nickel dibromide (9.8 mg, 0.02 mmol), potassium phosphate (84.8 mg, 0.4 mmol), 1,3-enyne compound 1a (47.1 mg, 0.24 mmol), 4-bromoethyl benzoate 2a (45.8 mg, 0.2 mmol), cyclohexane 3a (168.0 mg, 2 mmol) and acetone (0.5 mL). The reaction was carried out under the irradiation of a violet lamp (10w, 390nm) at room temperature for 12 hours, concentrated, and subjected to flash column chromatography to obtain the product 4 (71.9mg, 84% yield) as a colorless liquid.
[0026] 1 H NMR (600MHz, CDCl 3 )δ8.05(d,J=8.4Hz,2H),7.43-7.34(m,5H),7.31-7.28(m,2H),4.40(q,J=7.1Hz,2H),2.22(d,J =7.1Hz, 2H), 1.74-1.67(m, 2H), 1.64-1.60(m, 3H), 1.57-1.50(m, 1H), 1.40(t, J=7.1Hz, 3H), 1.19-1.08( m,3H),0.94-0.85(m,2H); 13 C NMR (151MHz, CDCl 3 )δ204.4(q,J=4.1Hz),166.2,139.7,134.3,130.1,129.8,128.7,128.5,...
Embodiment 2
[0028]
[0029] In an inert atmosphere, sodium decapolytungstate (13.3 mg, 0.004 mmol), (4,4'-di-tert-butyl-2,2'-bipyridine) nickel dibromide was added to the reaction tube containing the magnetron. (9.8 mg, 0.02 mmol), potassium phosphate (84.8 mg, 0.4 mmol), 1,3-enyne compound 1b (54.3 mg, 0.24 mmol), 4-bromoethyl benzoate 2a (45.8 mg, 0.2 mmol), Cyclohexane 3a (168.0 mg, 2 mmol) and acetone (0.5 mL). Under the irradiation of a violet lamp (10w, 390nm), the reaction was carried out at room temperature for 12 hours, concentrated, and subjected to flash column chromatography to obtain the product 5 (58.6 mg, 64% yield) as a colorless liquid.
[0030] 1 H NMR (600MHz, CDCl 3 )δ8.04(d,J=8.4Hz,2H),7.39(d,J=8.4Hz,2H),7.22(d,J=8.8Hz,2H), 6.91(d,J=8.8Hz,2H) ,4.39(q,J=7.1Hz,2H),3.83(s,3H),2.24-2.16(m,2H),1.74-1.67(m,2H),1.65-1.58(m,3H),1.57-1.49 (m, 1H), 1.40 (t, J=7.1Hz, 3H), 1.22-1.08 (m, 3H), 0.95-0.84 (m, 2H); 13 C NMR (151MHz, CDCl 3 )δ204.4(q,J=4.1Hz),166.4,159.9,140.2...
Embodiment 3
[0032]
[0033] In an inert atmosphere, sodium decapolytungstate (13.3 mg, 0.004 mmol), (4,4'-di-tert-butyl-2,2'-bipyridine) nickel dibromide was added to the reaction tube containing the magnetron. (9.8 mg, 0.02 mmol), potassium phosphate (84.8 mg, 0.4 mmol), 1,3-enyne compound 1c (53.1 mg, 0.24 mmol), 4-bromoethyl benzoate 2a (45.8 mg, 0.2 mmol), Cyclohexane 3a (168.0 mg, 2 mmol) and acetone (0.5 mL). Under the irradiation of a violet lamp (10w, 390nm), the reaction was carried out at room temperature for 12 hours, concentrated, and subjected to flash column chromatography to obtain 6 (22.3 mg, 25% yield) as a colorless liquid product.
[0034] 1 H NMR (600MHz, CDCl 3 )δ8.07(d,J=8.3Hz,2H),7.67(d,J=8.3Hz,2H),7.40(d,J=8.4Hz,2H), 7.34(d,J=8.4Hz,2H) ,4.40(q,J=7.1Hz,2H),2.23(d,J=7.1Hz,2H),1.65-1.60(m,5H),1.54-1.49(m,1H), 1.40(t,J=7.1 Hz,3H),1.16-1.08(m,3H),0.92-0.85(m,2H); 13 C NMR (151MHz, CDCl 3 )δ204.9(q,J=4.2 Hz),166.0,139.4,138.4,132.6,130.7,130.2,129.0,128.4,123.4(q,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com