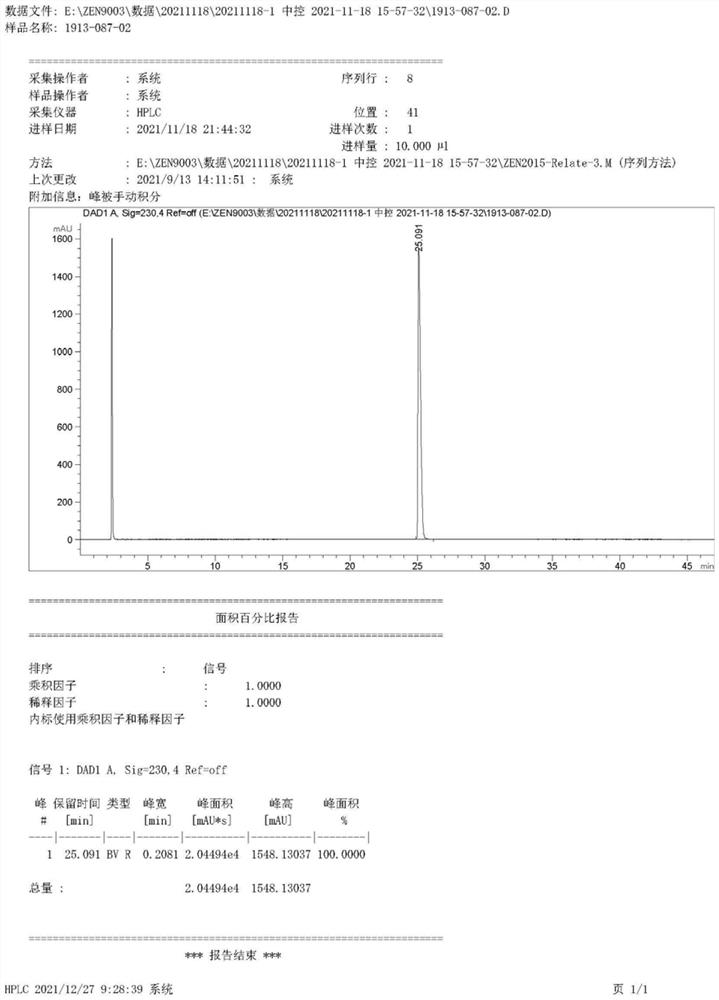
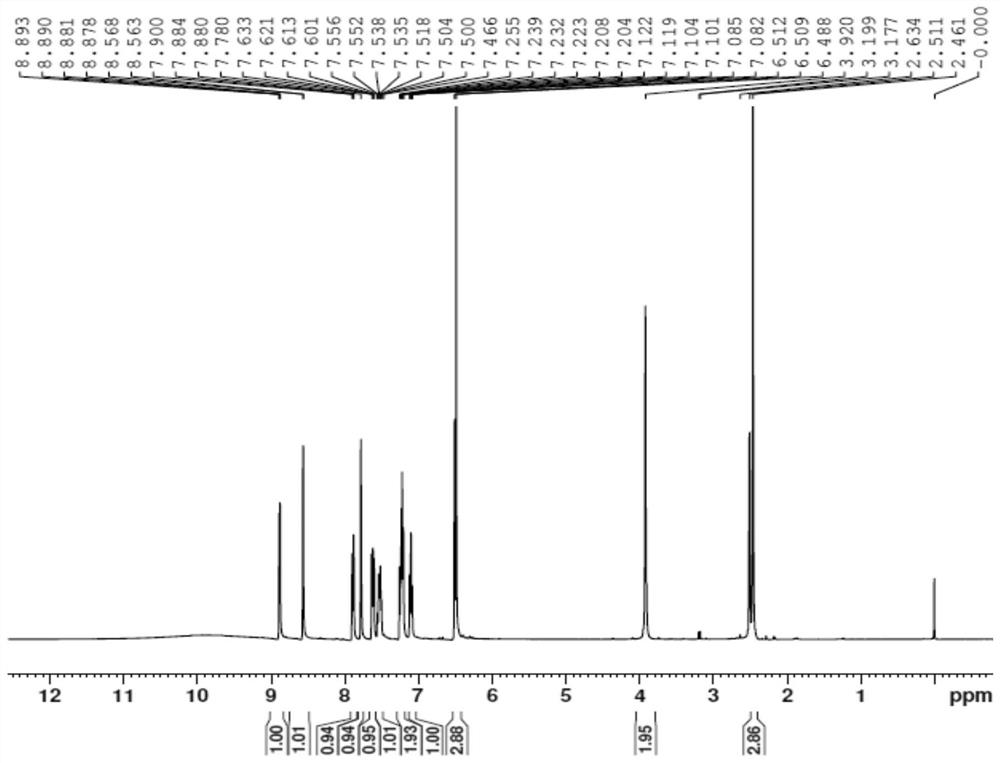
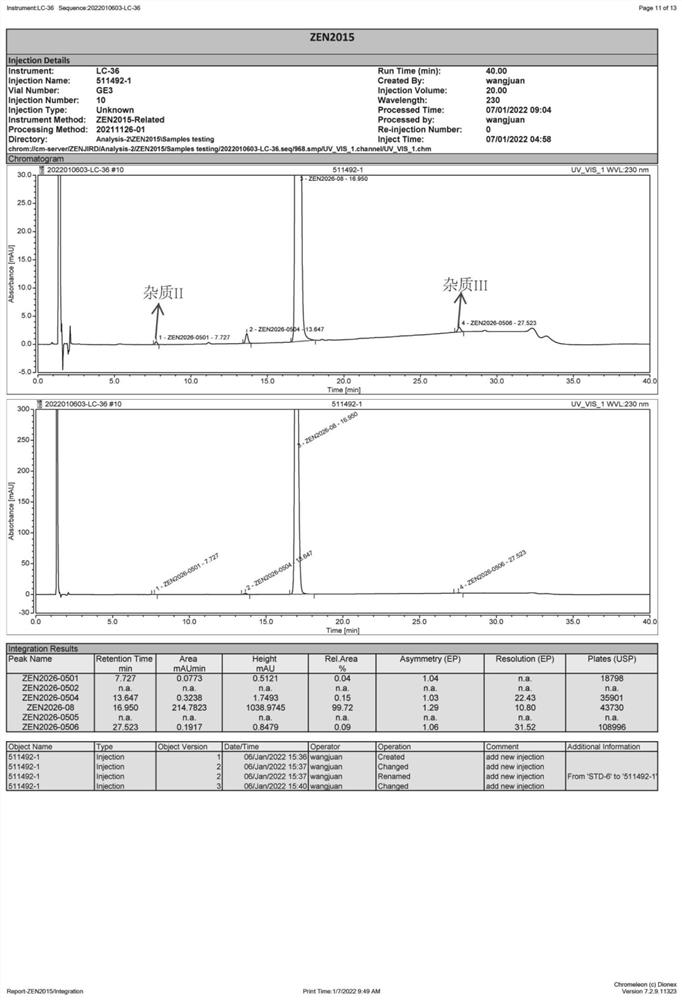
Preparation method of Vonoprazan fumarate
A technology of fumaric acid and dicarbonic acid, which is applied in the field of preparation of vonolax fumarate, can solve the problems of weakened safety, unfavorable industrialized large-scale production, impurity I and impurity II cannot be removed, and the like. effect, improved efficacy and safety, simple starting material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of compound 3
[0041]
[0042] 530.00g of acetonitrile, 200.00g of compound 1, 25.83g of DMAP, 117.67g of triethylamine were successively added to the 500ml reaction flask, the stirring was turned on, the temperature was controlled between 15 and 35°C, and 206.52g of compound 2 was added dropwise. The dropwise addition was completed in about 30min; at 15-35°C, the reaction was incubated for 1-3h; then, the reaction solution was added to 1000.00 g of water with a temperature of 5-15°C, stirred for 1-2 hours, filtered, and the filter cake was rinsed with water. , dried at 50°C to obtain 321.27g of brown solid, HPLC purity: 99.5%, yield 92%.
[0043] Preparation of compound 4
[0044]
[0045] Between 10 and 20°C, add 433.97g of 13% methylamine methanol solution, 400.00g of methanol and 200.00g of compound 3 into the reaction flask in turn, keep stirring for 1 to 2h; between -15 to 0°C , add 11.45g of sodium borohydride in batches, keep stirring for 1h...
Embodiment 2
[0057] Preparation of compound 3
[0058] Add 530.00g of tetrahydrofuran, 200.00g of compound 1, 25.83g of DMAP, and 150.29g of DIPEA into the 500ml reaction flask in turn, turn on stirring, control the temperature between 15 and 35°C, and add 206.52g of compound 2 dropwise for about 30 minutes. The left and right dropwise addition is completed; at 15-35 ℃, the reaction is incubated for 1-3 hours; then the reaction solution is added to 1000.00 g of water with a temperature of 5-15 ℃, stirred for 1-2 hours at the temperature, filtered, and the filter cake is rinsed with water and placed in After drying at 50°C, 320.22 g of a brown solid was obtained. The HPLC purity was 99.3%, and the yield was 91.7%.
[0059] Preparation of compound 4
[0060] Between 10 and 20°C, add 433.97g of 13% methylamine ethanol solution, 400.00g of ethanol and 200.00g of compound 3 into the reaction flask in turn, keep stirring for 1 to 2h; between -15 to 0°C , add 513.28g of sodium borohydride aceta...
Embodiment 3
[0069] Preparation of compound 3
[0070] 530.00g of methylene chloride, 200.00g of compound 1, 25.83g of DMAP, 123.26g of sodium carbonate were successively added to the 500ml reaction flask, stirring was turned on, the temperature was controlled between 15 and 35°C, and 206.52g of compound 2 was added dropwise. , the dropwise addition was completed in about 30min; at 15-35 ℃, the reaction was incubated for 1-3 hours; then the reaction solution was added to 1000.00 g of water with a temperature of 5-15 ℃, stirred for 1-2 hours, filtered, and the filter cake was rinsed with water After that, it was dried at 50° C. to obtain 319.27 g of brown solid, HPLC purity: 99.3%, yield 91.5%.
[0071] Preparation of compound 4
[0072] Between 10 and 20°C, add 433.97g of 13% methylamine methanol solution, 400.00g of tetrahydrofuran and 200.00g of compound 3 into the reaction flask in sequence, and keep stirring for 1 to 2h; at -15 to 0°C , add 26.63 g of sodium cyanoborohydride in batch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com