Construction of human-like collagen recombinant pichia pastoris engineering bacteria and rapid protein purification method
A technology of human-like collagen and recombinant collagen, which is applied in the fields of biotechnology and genetic engineering, can solve the problems of long purification process, low purity of collagen, environmental pollution, etc., and achieve simple, fast and cost-effective results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
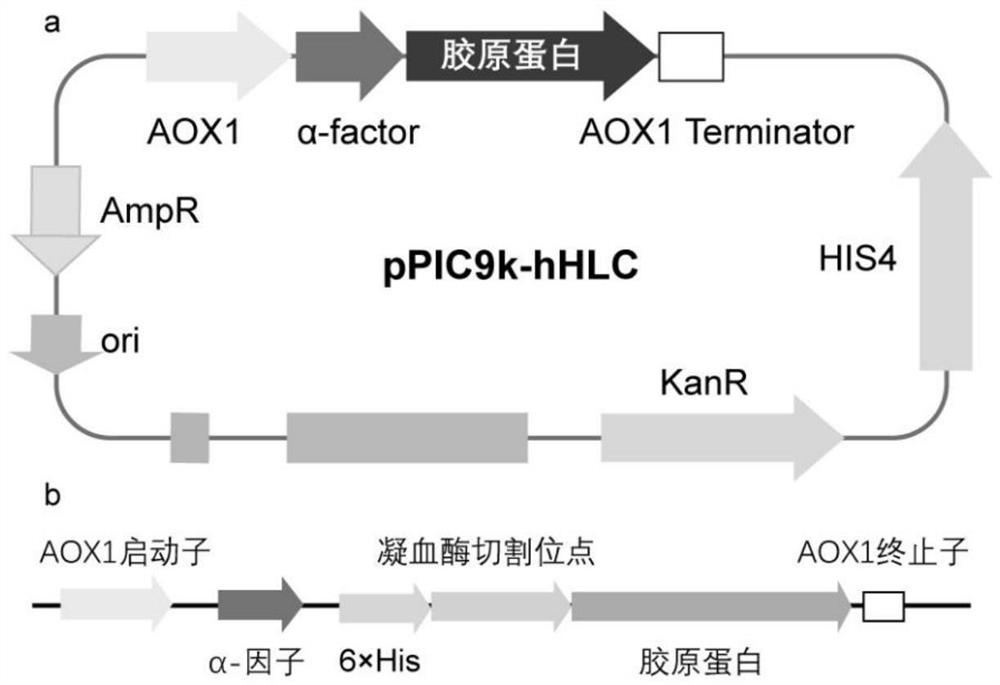
[0046] Example 1 Construction of recombinant expression vector pPIC9k-hHLC
[0047] Codon optimization was performed based on the sequence of SEQ ID NO: 1 to obtain a human-like collagen coding gene sequence of SEQ ID NO: 2, avoiding the SacI and SalI restriction sites, so as not to affect the subsequent transformation into Pichia pastoris. The sequence contains too many repetitive sequences, and it is not easy to use PCR amplification. Therefore, two sets of restriction sites were designed at both ends of the sequence (N-terminal added EcoRI and SacII restriction sites, C-terminal added PacI and NotI enzymes cleavage site) to facilitate the construction of this sequence into different expression vectors. Suzhou Jinweizhi Biotechnology Co., Ltd. was entrusted to conduct whole gene synthesis for the sequence of SEQ ID NO: 2.
[0048] The synthetic full gene sequence SEQ ID NO:2 was integrated into the pPIC9k vector through the two restriction sites of EcoRI and NotI, transform...
Embodiment 2
[0049] Example 2 Construction of recombinant genetically engineered strains
[0050] The recombinant expression vector pPIC9k-hHLC in Example 1 was linearized by cutting the recombinant expression vector pPIC9k-hHLC in Example 1 by SacI restriction endonuclease at 37°C for 1 h, and the linearized fragment was purified and recovered using a PCR purification kit to obtain pPIC9k-hHLC linearized fragment . The purified pPIC9k-hHLC linearized fragment was transformed into Pichia pastoris GS115 competent cells, spread on MD plates, and cultured at 30°C for 3-5 days. Select positive clones on YPD plates containing different concentrations of G418, culture at 30°C for 3-5 days, select positive clones that can grow at different concentrations for induction expression, and obtain recombinant Pichia engineering bacteria with multiple copies. . The specific method is as follows:
[0051] 1) Inoculate the activated Pichia GS115 on the YPD plate in 25mLYPD medium / 250mL conical flask, an...
Embodiment 3
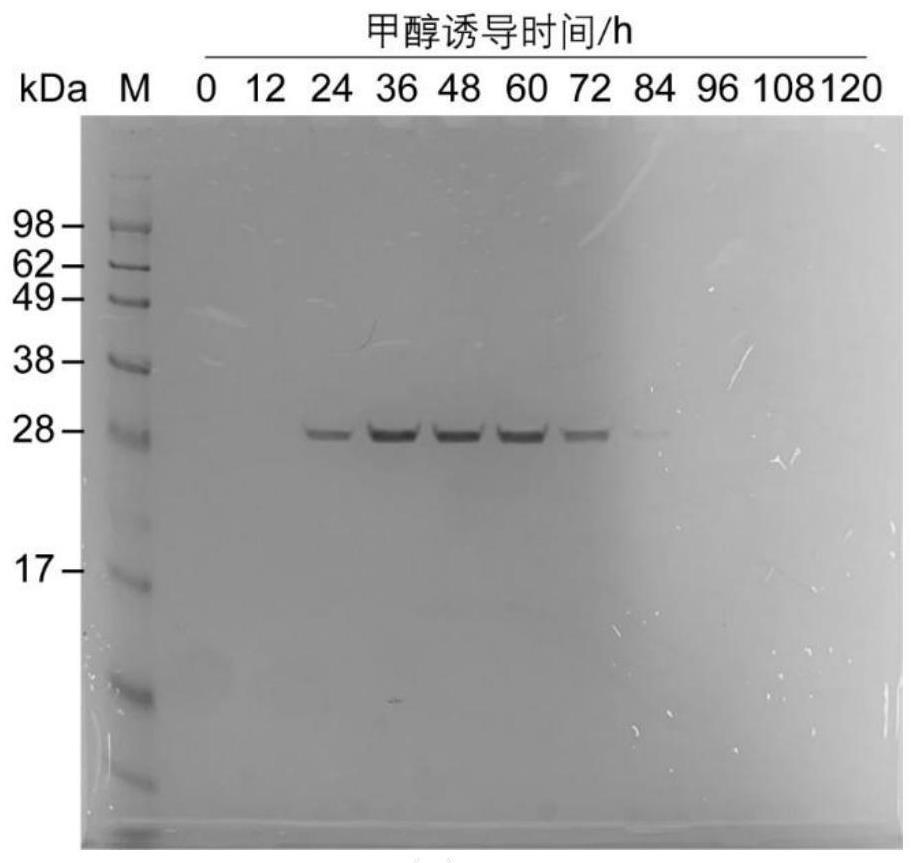
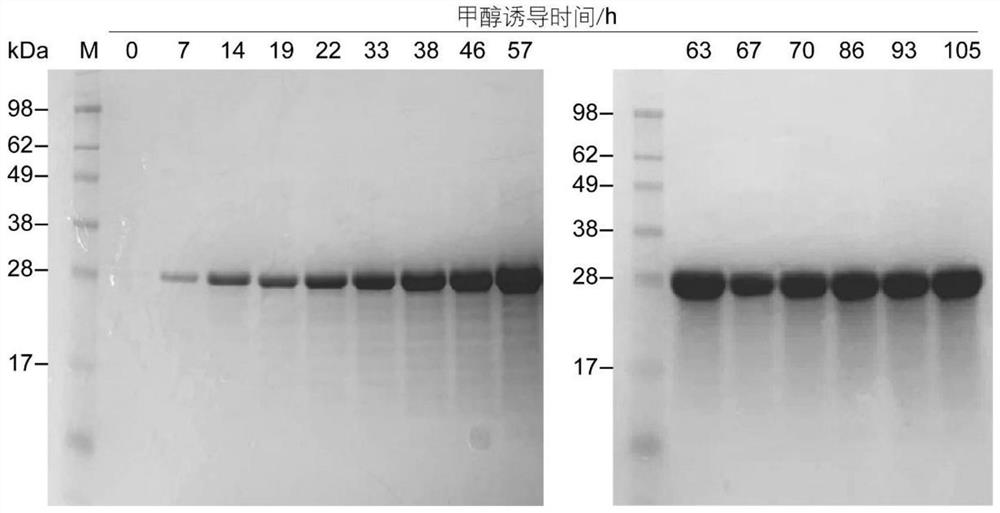
[0058] Example 3 Shake flask fermentation of recombinant Pichia pastoris
[0059] The high-copy transformant GS115 / pPIC9k-hHLC grown on the YPD plate of 4 mg / mL G418 was picked, inoculated into a 250 mL Erlenmeyer flask containing 50 mL of YPD, and cultured at 30 °C and 220 rpm for 16-20 h to obtain a strain activation solution. Add 500 μL of bacterial activation solution to 50 mL of BMGY medium, and cultivate it to the OD of the bacterial suspension at 30 °C and 220 rpm. 600 For 2-6, centrifuge at 5000rpm for 10min, collect the cells and resuspend in BMMY medium to dilute the OD to about 1. The resuspended bacterial suspension was placed at 28° C. and 220 rpm to induce expression, and methanol with a final concentration of 0.5% (V / V) was supplemented every 24 h for continuous induction. After 72h-96h of methanol-induced culture, centrifuge at 5000rpm for 10min, and collect the supernatant. The protein expression in the fermentation supernatant was detected by SDS-PAGE ( f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com