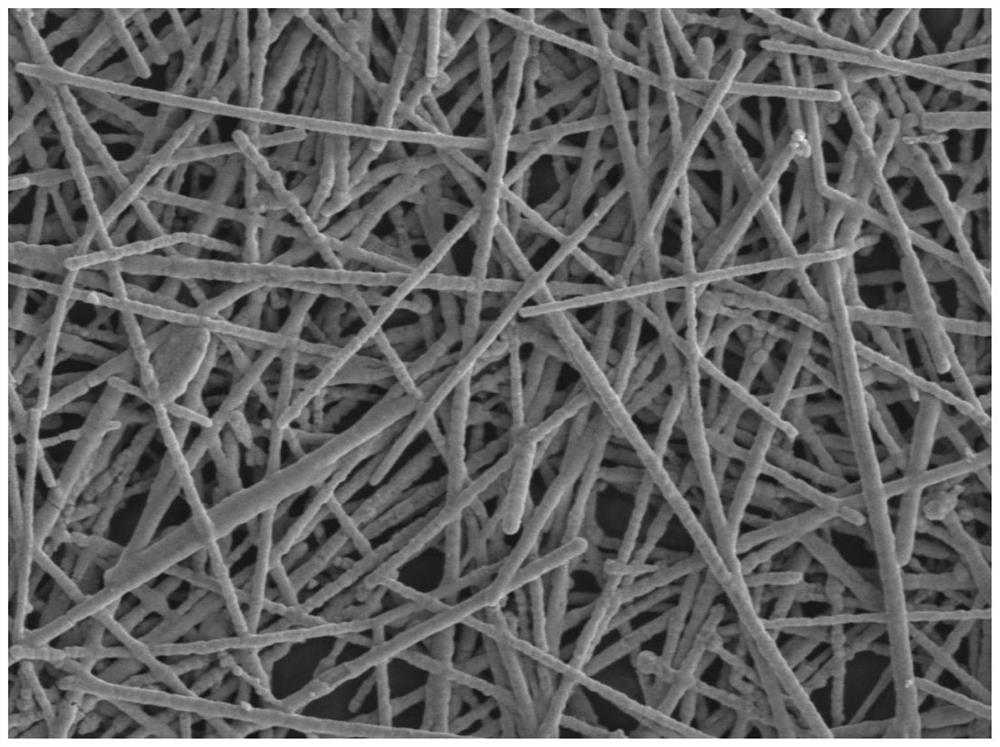
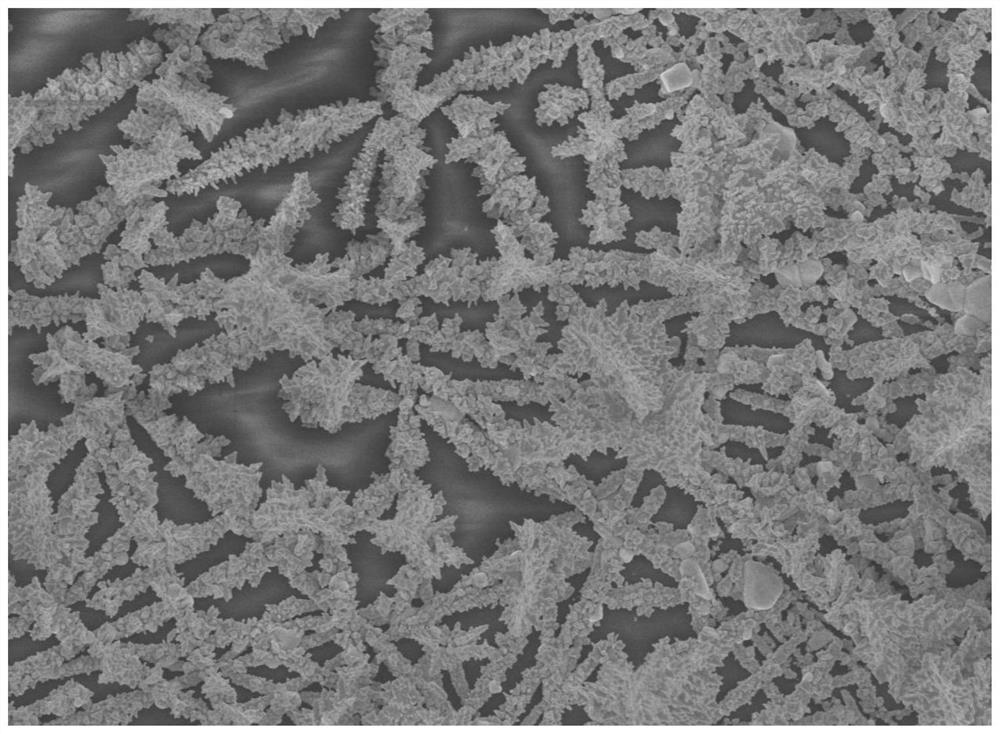
Method for constructing neutral glucose fuel cell electrode
A fuel cell electrode and glucose technology, which is applied in the field of electrochemical detection, can solve the problems that the oxidation performance is easily affected by pH, limits the performance and life of the electrocatalyst, and does not have the ability to catalyze glucose, and achieves good long-term stability and good catalytic effect , the effect of high electrochemical response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 Glucose solution and blank PBS solution cyclic voltammetry curve comparison
[0054] First, the three-electrode system was placed in a PBS solution with a pH of 7.4 and a concentration of 0.1 M, and cyclic voltammetry was used to scan in the potential range of -0.7V to 1.0V, and the cyclic voltammetry curve of the blank solution was recorded; then , the three-electrode system was placed in a 30 mM glucose test solution containing 0.1 M PBS solution with a pH of 7.4 as a supporting electrolyte, and cyclic voltammetry was used to scan in the potential range of -0.7 V to 1.0 V to record glucose. cyclic voltammetry curve.
[0055] The result is as Figure 4 Shown: The catalytic effect of the Pd-Au-PDMS electrode in 30 mM glucose was tested at a scan speed of 50 mV / s. It can be seen from the figure that the catalytic current of Pd-Au-AgNWs electrode towards glucose is 11900 μA / cm 2 / mol. It is shown that the fuel composed of Pd-Au-AgNWs electrode can efficie...
Embodiment 2
[0057] In 0.1M PBS solution, Pd-Au-AgNWs electrode, Pd-AgNWs electrode and Pd-AuNWs electrode were subjected to cyclic voltammetry responses in 30 mM glucose solution, respectively.
[0058] The three-electrode system was sequentially placed in different concentrations of glucose to be tested containing 0.1 M PBS solution with a pH of 7.4 as the supporting electrolyte, and the current curve of 30 mM glucose was measured at a scan rate of 50 mV / s. Anfa, scan in the potential range of -0.7V ~ 1.0V. The cyclic voltammetry curves of Pd-Au-AgNWs electrode, Pd-AgNWs electrode and Pd-AuNWs electrode with the same concentration and scan rate of glucose were recorded.
[0059] The result is as Figure 5 Shown: It can be seen from the figure that the Pd-Au-AgNWs electrode has the highest electrochemical activity in the glucose solution. Compared with the Pd-AgNWs electrode and the Pd-AuNWs electrode, the peak current density of the Pd-Au-AgNWs electrode is greatly improved, which can ...
Embodiment 3
[0061] Cyclic voltammetry responses of Pd-Au-AgNWs electrodes to different glucose solutions with the same concentration in 0.1M PBS solution.
[0062] The three-electrode system was sequentially placed in different concentrations of glucose to be tested containing 0.1M PBS solution with pH of 7.4 as the supporting electrolyte, and the measured concentrations were 10mM, 20mM, 30mM, 40mM, 50mM at a scan rate of 50mV / s. , 60 mM, 70 mM, 80 mM, 90 mM, 100 mM glucose current curves were scanned in the potential range of -0.7V to 1.0V by cyclic voltammetry. The cyclic voltammetry curves of glucose at different concentrations and scan rates were recorded.
[0063] The result is as Image 6 , Figure 7 Shown: It can be seen from the figure that as the concentration increases, the oxidation current of the nanoelectrode in the glucose solution also increases, and the oxidation peak also increases, showing a good linear response of catalyzing glucose. Therefore, the redox reaction of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com