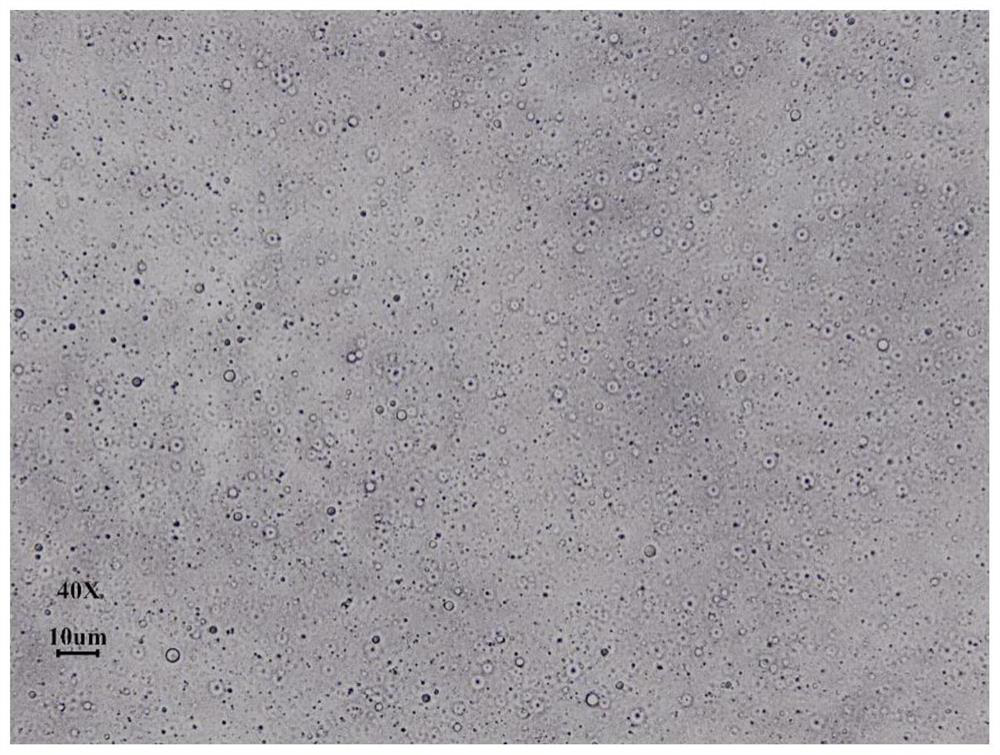
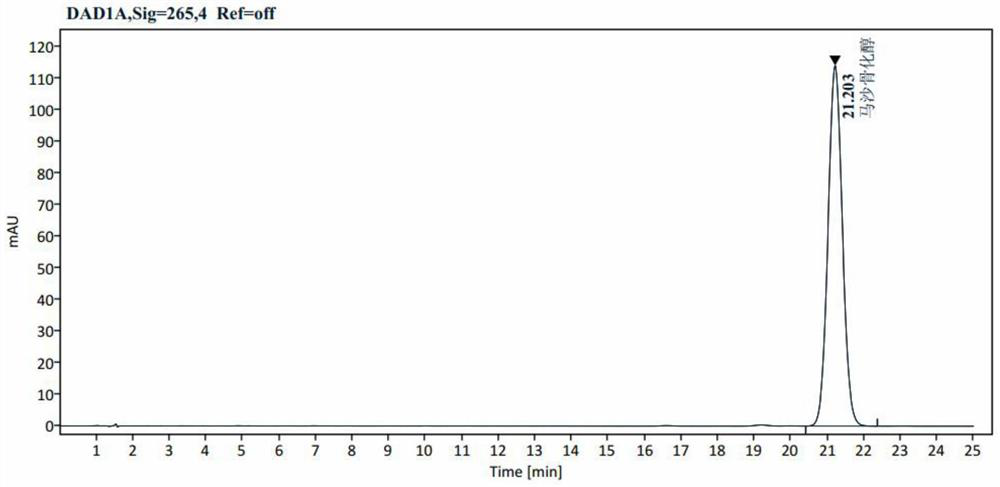
Suspension gel for treating inflammatory dermatosis
An inflammatory skin disease and gel technology, applied in the field of medicinal chemistry, can solve the problems of chemical stability risk of maxacalcitol, thermal instability of maxacalcitol, and poor compliance of patients with medication, etc. Effects of tack and elasticity, easy spread and wash off, excellent physical and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] This embodiment provides a suspension gel, the total mass of the suspension gel is 500g, and the raw and auxiliary materials for the preparation of the suspension gel include the following components in terms of mass percentage:
[0104]
[0105] The preparation method of the suspension gel comprises the following steps:
[0106] (1) Dissolve maxacalcitol in the mixed solvent of caprylic acid capric triglyceride, isopropyl myristate, light liquid paraffin and diethylene glycol monoethyl ether according to the recipe quantity, then add dibutyl Hydroxytoluene, dl-α-tocopherol, Span 80 and polyoxyethylene-35 castor oil were mixed at 25°C for 2h to obtain an oil phase;
[0107] (2) mix Poloxamer 188 and purified water at 25°C for 0.5h according to the recipe to obtain an aqueous phase;
[0108] (3) Disperse Carbomer Tr-1 in water according to the recipe quantity, add triethylamine alcohol to adjust pH to 6.0 after complete swelling, and mix for 8h to obtain a gel phase;...
Embodiment 2
[0111] This embodiment provides a suspension gel, the total mass of the suspension gel is 1000g, and the preparation raw and auxiliary materials of the suspension gel include the following components in terms of mass percentage:
[0112]
[0113] The preparation method of the suspension gel comprises the following steps:
[0114] (1) Dissolve maxacalcitol in the mixed solvent of caprylic acid capric triglyceride, isopropyl myristate, light liquid paraffin and diethylene glycol monoethyl ether according to the recipe quantity, then add dibutyl Hydroxytoluene, dl-α-tocopherol, Span 80 and polyoxyethylene-35 castor oil were mixed at 25°C for 2h to obtain an oil phase;
[0115] (2) mix Poloxamer 188 and purified water at 25°C for 0.5h according to the recipe to obtain an aqueous phase;
[0116] (3) Disperse Carbomer Tr-1 in water according to the recipe quantity, add triethylamine alcohol to adjust pH to 6.0 after complete swelling, and mix for 8h to obtain a gel phase;
[01...
Embodiment 3
[0119] This embodiment provides a suspension gel, the total mass of the suspension gel is 1000g, and the preparation raw and auxiliary materials of the suspension gel include the following components in terms of mass percentage:
[0120]
[0121] The preparation method of the suspension gel comprises the following steps:
[0122] (1) Dissolve maxacalcitol in the mixed solvent of caprylic acid capric triglyceride, isopropyl myristate, light liquid paraffin and diethylene glycol monoethyl ether according to the recipe quantity, then add dibutyl Hydroxytoluene, dl-α-tocopherol, Span 80 and polyoxyethylene-35 castor oil were mixed at 25°C for 2h to obtain an oil phase;
[0123] (2) mix Poloxamer 188 and purified water at 25°C for 0.5h according to the recipe to obtain an aqueous phase;
[0124] (3) Disperse Carbomer Tr-1 in water according to the recipe quantity, add sodium hydroxide to adjust pH to 6.0 after complete swelling, and mix for 8h to obtain a gel phase;
[0125] (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com