Application of nicotinic glycoside or analogue thereof in preparation of medicine for preventing and treating traumatic brain injury
A technology of nicotinic glycosides and analogs, which is applied in the field of application of nicotinic glycosides or their analogs in the preparation of drugs for the prevention and treatment of traumatic brain injury, which can solve the problems of no reports, etc., and achieve improvement of exercise and reduction of oxidative stress in brain tissue The effect of stimulating and inflammatory factor levels and reducing the degree of cerebral edema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of nicotinoid and its analogues
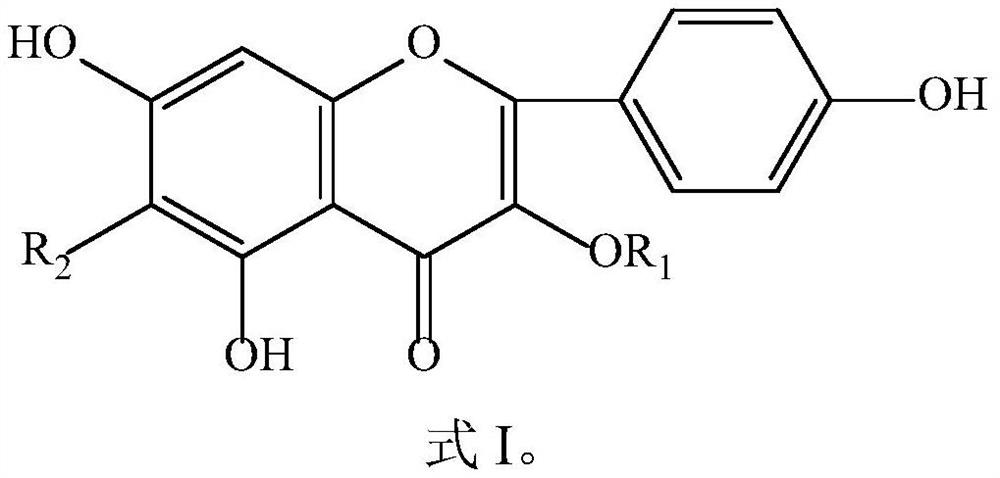
[0035] Preparation of kaempferol, 6-hydroxykaempferol, kaempferol-3-glucoside, kaempferol-3-rutinoside, 6-hydroxykaempferol-3-glucoside, 6-hydroxykaempferol-3 , 6-diglucoside, 6-hydroxykaempferol-3,6,7-triglucoside.
[0036] 35kg of Hefei safflower dried medicinal materials were soaked in 117L of 80% ethanol at room temperature for 48 hours, extracted three times, the extracts were combined, and the ethanol was recovered under reduced pressure to obtain a concentrated solution. The petroleum ether extraction part was discarded, and the ether and n-butanol extraction parts were respectively concentrated under reduced pressure and then subjected to column chromatography one by one.
[0037] 1. The ether extract was subjected to silica gel column chromatography, and eluted with petroleum ether-ethyl acetate (30:1-1:1) and chloroform-methanol (10:1-1:1) solvent system as usual (the same below). ), respectively colle...
Embodiment 2
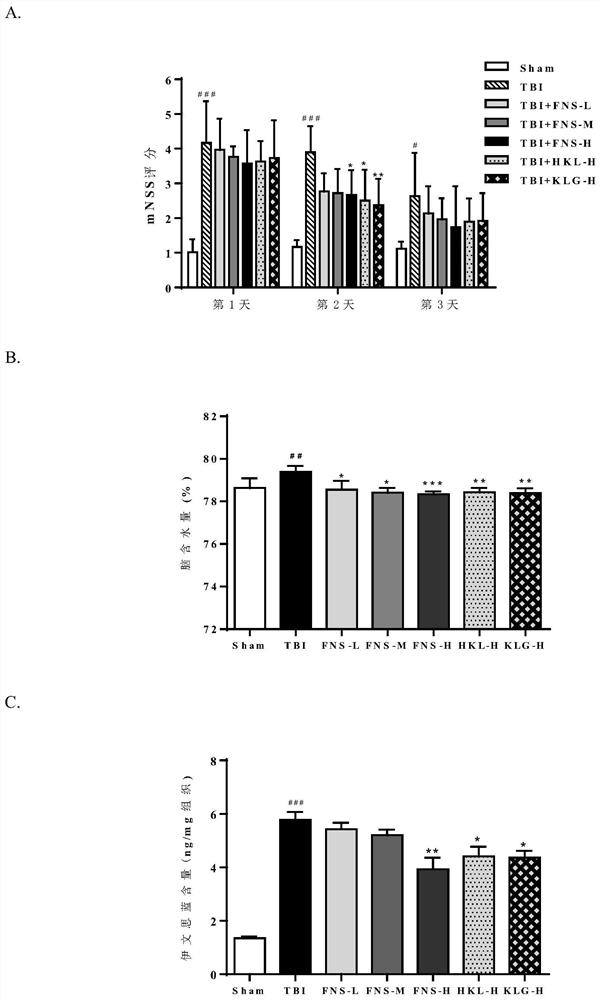
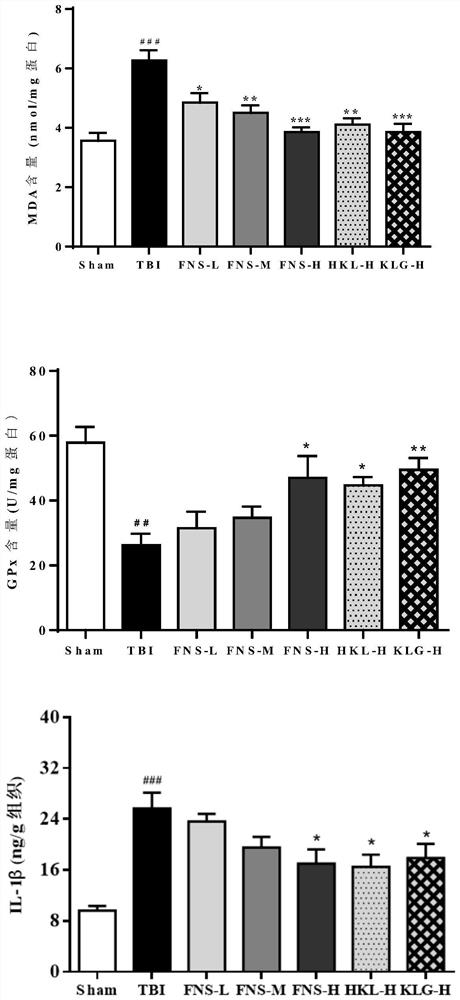
[0042] Example 2: Protective effects of FNS, HKL and KLG on closed diffuse brain injury in rats induced by weight-dropping method
[0043] 1. Test material
[0044] 1.1 Experimental instrument
[0045] Iron stand, PVC pipe, weights.
[0046] 1.2 Test samples
[0047] The purity of pentobarbital sodium, 0.9% saline, FNS, HKL and KLG were all greater than 95.2%. Preparation: Dissolve HKL and KLG in ethanol before use: Tween-80:H 2 O is in a solvent of 1:1:8 for use.
[0048] 2. Experimental animals
[0049] Strain: Male Wistar rat, 260-290 g
[0050] Feeding: constant temperature purification and ventilation animal room, free food and water, 25 ± 2 ℃.
[0051] 3. Grouping and administration
[0052] 84 rats were randomly divided into 7 groups, namely: sham operation group (1), TBI group (2), FNS-L (3), FNS-M (4), FNS-H (5), KLH-H ( 6), KLG-H (7). Among them, the doses corresponding to L, M, and H were 2.5, 5.0, and 10.0 mg / kg / d in the drug treatment groups after TBI mo...
Embodiment 3
[0063] Example 3: Protective effects of FNS, HKL and KLG on brain injury induced by hydraulic shock model in rats
[0064] 1. Test material
[0065] 1.1 Experimental instrument
[0066] STRONG-90 dental drill, ALC-FP6 hydraulic percussion instrument (Alcott Biotech, Shanghai), thermal insulation heating pad, Tissuclyscr-48 multi-sample tissue grinder (Shanghai Jingxin Co., Ltd.), 10S UV spectrophotometer (ThermoFisher Scientific), Pico 17 high-speed Centrifuge (Thermo Fisher Scientific).
[0067] 1.2 Test samples
[0068] Evans blue, formamide (Dalian Meilun Biotechnology Co., Ltd.).
[0069] 2. Experimental animals
[0070] Same as Example 2, the number of animals is required for each part of the experiment.
[0071] 3. Grouping and administration
[0072] Same as Example 2.
[0073] 4. Test method
[0074] 4.1 TBI model establishment
[0075] After being anesthetized with sodium pentobarbital by intraperitoneal injection, the rats were fixed on the heating pad of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com