Intramolecular sensitized macromolecular photoinitiator containing pyrazoline and alpha-aminoketone as well as preparation method and application of intramolecular sensitized macromolecular photoinitiator
A technology of photoinitiator and pyrazoline, applied in the field of intramolecular sensitized macromolecular photoinitiator and its preparation, can solve problems such as toxicity and limitation, and achieve the effects of low volatility, reduced odor and low mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Preparation of α-aminoketone-based small molecule photoinitiator PI-2 containing double bonds
[0073]
[0074] 30.9 g of 2-morpholino-4'-(2-hydroxyethylthio)-2-methylpropiophenone (0.1 mol) and 300 ml of n-heptane were added to the double-layer reaction flask, and then 2-morpholine was added. In the mixed solution of base-4'-(2-hydroxyethylthio)-2-methylpropiophenone and n-heptane; add 12 grams of 50% aqueous sodium hydroxide solution dropwise to the double-layer reaction flask and 0.3 g of tetrabutyl ammonium bromide, turn on the condensed water, control the reaction temperature at room temperature 25 ° C, under the protection of nitrogen atmosphere, dropwise add the n-heptane solution of 50 ml of allyl bromide, the allyl bromide is 0.12mol After the dropwise addition, the temperature of the reaction system will rise immediately, control the dropwise temperature to be below 30°C, and finish dropping in about 30 minutes, and then stir the reaction at room...
Embodiment 2
[0076] Example 2: Preparation of α-aminoketone-based small molecule photoinitiator PI-1 containing double bonds
[0077]
[0078] 30.9 g of 2-morpholinyl-4'-(2-hydroxyethylthio)-2-methylpropiophenone (0.1 mol) and 300 ml of anhydrous dichloromethane were added to the double-layer reaction flask, followed by 12.2 g of Triethylamine (0.12mol), turn on the low temperature cycle, lower the temperature in the double-layer reaction flask to 0°C, and under the protection of nitrogen atmosphere, add methanesulfonyl chloride (0.105mol) or p-toluene dropwise to the double-layer reaction flask 50 ml of dichloromethane solution of sulfonyl chloride (0.105mol), the temperature of the reaction system will rise immediately, control the dropwise temperature to be below 5 ℃, and the dropwise addition is completed in about 30 minutes, then stir at low temperature for 30 minutes, and then rise to room temperature and react for 2 hours , monitor the reaction by spot plate to the end of the rea...
Embodiment 3
[0085] Embodiment 3: Utilize thiol-ene click reaction to prepare target product photoinitiator 1
[0086]
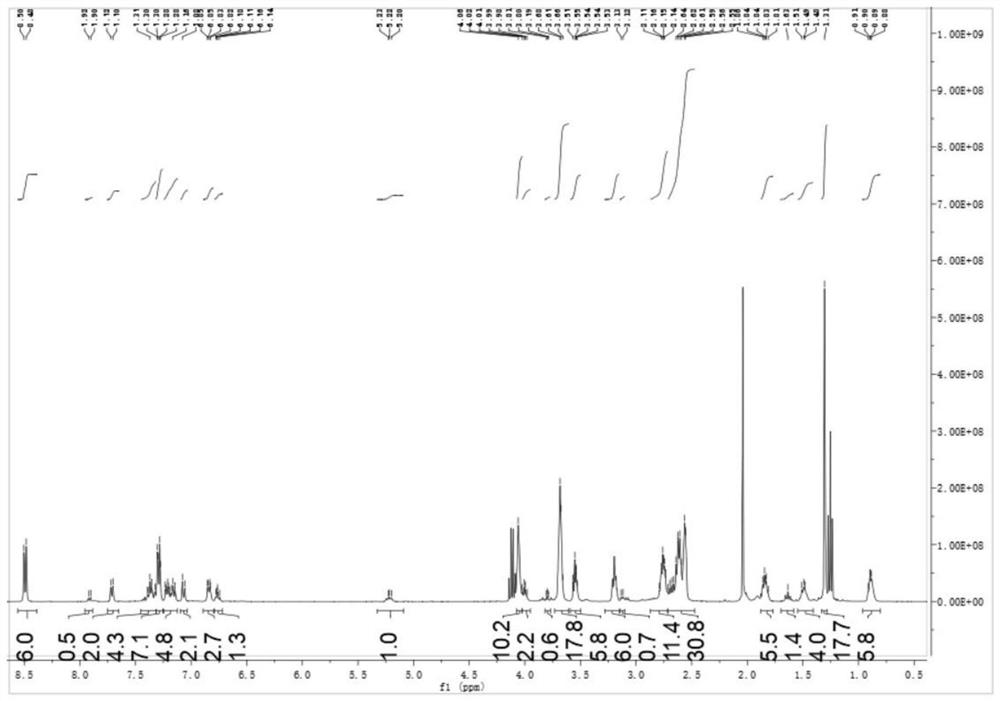
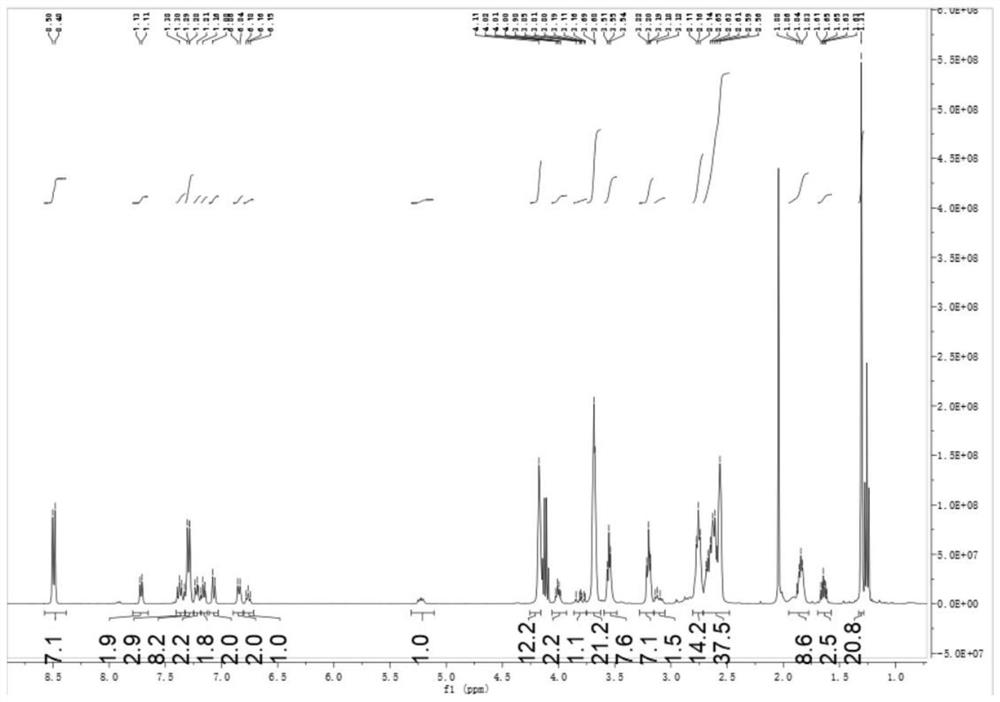
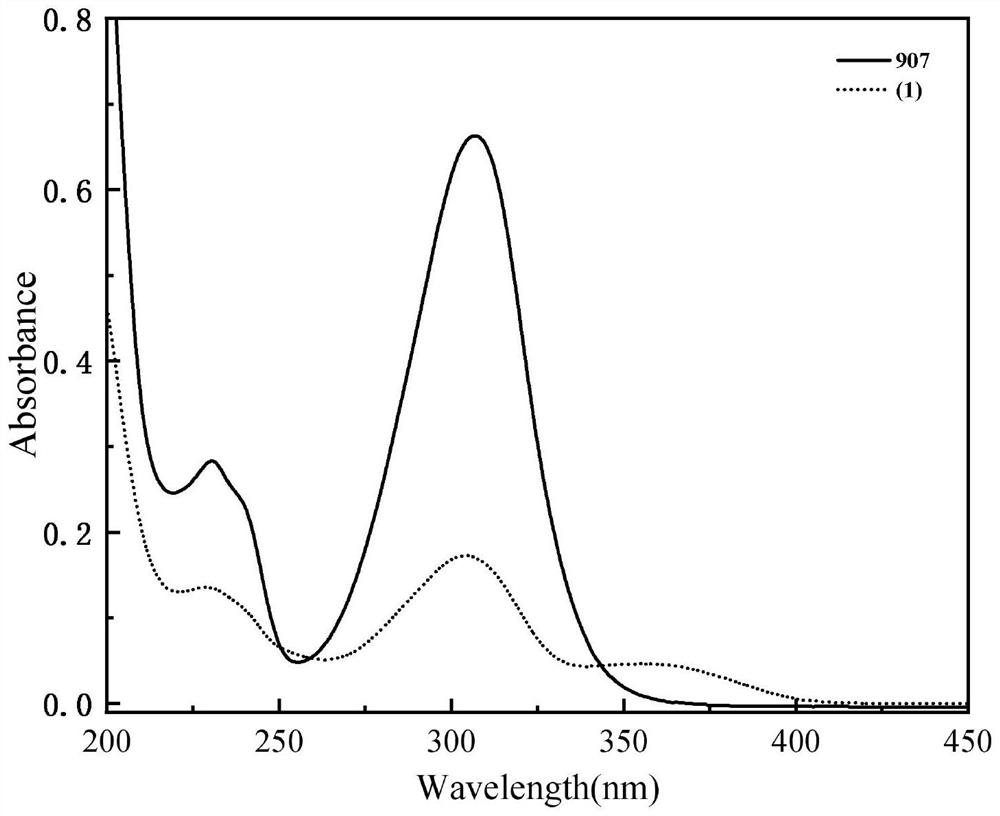
[0087] Add 6.98 g (20 mmol) of photoinitiator PI-2 and 3.54 g (10 mmol) of pyrazoline derivative A containing double bonds to the three-necked flask of 100 ml, then add 3.98 g (10 mmol) of trithiol and azobis 0.180 g of isobutyronitrile, evacuated, filled with nitrogen for three cycles, placed the three-necked flask in an oil bath at 50 °C, stirred for 30 minutes, heated to 70 °C, reacted for 2 hours, and then reacted at 90 °C for 1 hour; Spectral monitoring found 2570cm -1 The peak of thiol disappears, and the reaction ends after thin-plate chromatography is free of raw materials; α-aminoketone small molecule photoinitiator PI containing double bond, pyrazoline derivative A containing double bond and polythiol compounds B was prepared by the thiol-alkene click reaction to prepare the target product photoinitiator 1.
[0088] After the reaction is completed, it is not...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com