Application of metal nitrogen-based hydrogen storage material in hydrogen isotope separation and preparation method of metal nitride
A technology for hydrogen isotopes and hydrogen storage materials, applied in the separation of different isotopic elements, separation methods, nitrogen-metal/silicon/boron binary compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Weigh 2g Li rods with a purity of 99.8% in a glove box protected by an inert gas, put them into a ball mill jar, and fill the jar with high-purity nitrogen gas of 10MPa. , the speed is 400 rpm. After the ball milling, the high-purity nitrogen gas was slowly filled into the ball mill tank at room temperature to 10MPa, and the nitrogen gas was repeatedly filled until the pressure in the ball mill tank no longer decreased, and the reaction was carried out for a total of 40h to obtain brown Li. 3 N powder.
[0068] figure 1 is the obtained Li 3 XRD pattern of N sample, it can be seen that the reaction product is completely Li 3 N, no impurity phase is formed.
Embodiment 2
[0070] In an inert gas protection glove box, weigh 2g of LiAl alloy block with a purity of 98% and put it into a ball mill jar, and fill the jar with high-purity nitrogen gas of 10MPa. The ratio of ball to material is 60:1. hour at 400 rpm. After the ball milling, the ball mill tank was slowly filled with high-purity nitrogen to 10MPa at room temperature, and nitrogen was repeatedly filled until the pressure in the ball mill tank no longer decreased, and the reaction was carried out for a total of 60h to obtain gray-brown Li. 3 Mixed powder of N and AlN.
Embodiment 3
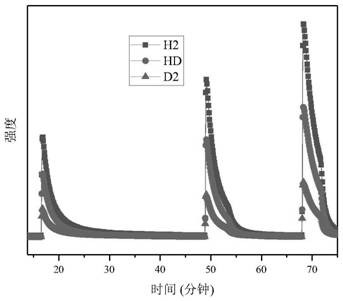
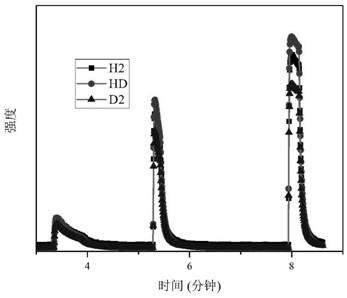
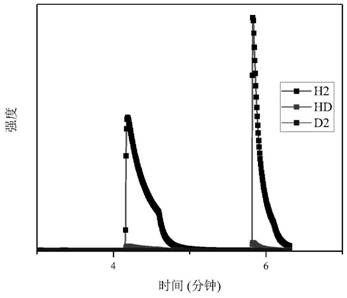
[0072] Weigh 0.14 g of Li in an inert gas protected glove box 3 N was loaded into the reactor, the reactor was evacuated and heated to 473K, and kept for 0.5 hours; by controlling the input gas pressure 0.2MPa, 0.0042mol of hydrogen isotope mixed gas (n D : n H = 1.8), after the adsorption is balanced (the pressure is no longer reduced), record the current pressure value, use the mass spectrometer to test the gas composition in the current reactor, and combine the total amount of hydrogen-deuterium mixture adsorbed by the solid material to obtain the hydrogen in the current material. Deuterium content molar ratio, recorded as [D] / [H]) solid ; The temperature of the reactor is quickly reduced to 293K, and the reactor is quickly evacuated to remove the gas mixture in the reactor that is not adsorbed; After the mixed gas in the reactor is completely evacuated, the reactor is slowly heated to make it warm to 473K And keep the temperature for 1 hour, thermally remove the mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com