Preparation method of avibactam sodium
A technology of avibactam sodium and a synthesis method, which is applied in the field of preparation of avibactam sodium, can solve the problems of long reaction time, volatile ammonia, and high reduction risk, and achieves simple operation, increased safety factor, and reduced production. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
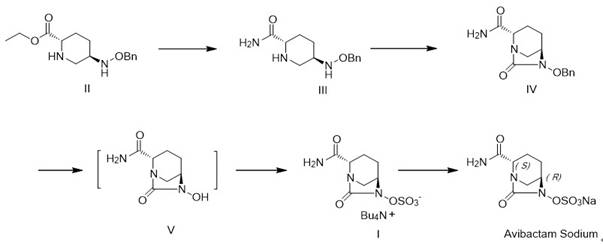
[0048] (1) Preparation of compound III
[0049] The starting material (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethyl ester oxalate 200 g (0.543 mol), methanol 2L, ammonium carbonate 156.6g was added to a 3L three-necked flask (1.63 mol, 3eq), react at 20-30°C for 10h, filter, concentrate the filtrate under reduced pressure to dryness (vacuum degree≤-0.08 Mpa), add 1L toluene at 80°C for 1h, and filter. Dry the wet product under vacuum at 45°C for 10h (vacuum degree≤-0.09 Mpa). 121.7 g of compound III were obtained with a yield of 90% and a purity of 99.6%.
[0050] (2) Preparation of compound IV
[0051] The reaction system was always under nitrogen protection. To 120 g (0.481 mol) of compound III, 0.5 L of chlorobenzene and 69.5 g (0.538 mol) of N,N-diisopropylethylamine were added, and then fluorene methoxycarbonyl chloride (138.9 g, 0.538 g) was added dropwise at a temperature of 10-15 °C. mol) solution in chlorobenzene (0.5L), heated to 25-30 °C for 3 hours...
Embodiment 2
[0059] (1) Preparation of compound III
[0060] Add starting material (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethyl ester oxalate 50g (0.14 mol), methanol 0.5L, ammonium chloride 7.5L into a 1L three-necked flask g (0.14 mol, 1eq), react at 20-30°C for 10h, filter, concentrate the filtrate under reduced pressure to dryness (vacuum degree≤-0.08 Mpa), add 1L toluene at 80°C for 1h, and filter. Dry the wet product under vacuum at 45°C for 10h (vacuum degree≤-0.09 Mpa). 32.4 g of compound III was obtained with a yield of 93% and a purity of 99.8%.
[0061] (2) Preparation of compound IV
[0062] The reaction system was always under nitrogen protection. To 32 g (0.13 mol) of compound III, 0.2 L of chlorobenzene, 18.8 g (0.14 mol) of N,N-diisopropylethylamine were added, and fluorene methoxycarbonyl chloride (37.1 g, 0.14 mol) was added dropwise at a temperature of 10-15 °C. mol) in 0.5L of chlorobenzene solution, heated to 25-30°C and reacted for 3 hours, temperatu...
Embodiment 3
[0070] (1) Preparation of compound III
[0071] Add starting material (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethyl ester oxalate 50g (0.14 mol), methanol 0.5L, ammonium bicarbonate 110 to a 1L three-necked flask g (1.4 mol, 10eq), react at 20-30°C for 2h, filter, concentrate the filtrate under reduced pressure to dryness (vacuum degree≤-0.08 Mpa), add 1L toluene at 80°C for 1h, and filter. Dry the wet product under vacuum at 45°C for 10h (vacuum degree≤-0.09 Mpa). 32.5 g of compound III was obtained with a yield of 96% and a purity of 99.4%.
[0072] (2) Preparation of compound IV
[0073] The reaction system was always under nitrogen protection. To 32 g (0.13 mol) of compound III, 0.2 L of chlorobenzene, 18.8 g (0.14 mol) of N,N-diisopropylethylamine were added, and fluorene methoxycarbonyl chloride (37.1 g, 0.14 mol) was added dropwise at a temperature of 10-15 °C. mol) in 0.5L of chlorobenzene solution, heated to 25-30°C for 3 hours, and the temperature wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com